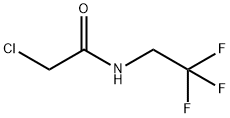
2-chloro-N-(2,2,2-trifluoroethyl)acetamide synthesis
- Product Name:2-chloro-N-(2,2,2-trifluoroethyl)acetamide
- CAS Number:170655-44-4
- Molecular formula:C4H5ClF3NO
- Molecular Weight:175.54
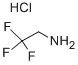
373-88-6

79-04-9

170655-44-4
GENERAL STEPS: 30.4 g (759.3 mmol) of sodium hydroxide and 50 mL of water were added to a 500 mL four-necked flask and stirred at room temperature until completely dissolved, then cooled to 5 °C. A solution was prepared by dissolving 50 g (370.4 mmol) of 2,2,2-trifluoroethylamine hydrochloride in 60 mL of water and added slowly and dropwise to the above sodium hydroxide solution at 5 °C. 85 mL of tert-butyl methyl ether was added and stirred continuously for 30 minutes. Subsequently, a solution of 43.9 g (388.9 mmol) of chloroacetyl chloride dissolved in 15 mL of tert-butyl methyl ether was added slowly dropwise while maintaining 5°C. The reaction mixture was heated to 10 °C and stirring was continued for 1 hour. After confirming complete consumption of 2,2,2-trifluoroethylamine by gas chromatography, the reaction mixture was warmed up to room temperature and partitioned. The aqueous layer was extracted with 100 mL of tert-butyl methyl ether, all organic layers were combined, and the solvent was concentrated by distillation under reduced pressure to give a tert-butyl methyl ether solution of 2-chloro-N-(2,2,2-trifluoroethyl)acetamide. The product was analyzed by high performance liquid chromatography with internal standard method to give 65 g of product in 100% yield and a solution concentration of 25.1% by weight. The solution was added dropwise to heptane to induce crystallization, and the melting point of the resulting 2-chloro-N-(2,2,2-trifluoroethyl)acetamide was 53.8 °C (as determined by DSC).
373-88-6
373 suppliers
$5.00/1g

79-04-9
382 suppliers
$12.00/5g

170655-44-4
74 suppliers
$38.00/100mg
Yield:170655-44-4 100%
Reaction Conditions:
Stage #1: 2,2,2-trifluoro-ethylamine hydrochloridewith tert-butyl methyl ether;sodium hydroxide in water monomer at 20; for 0.5 h;
Stage #2: chloroacetyl chloride at 5 - 10; for 1 h;
Steps:
1 Preparation of 2-chloro-N- (2,2,2-trifluoroethyl) acetamide
To a 500 ml four-necked flask, 30.4 g (759.3 mmol) of sodium hydroxide and 50 g of water were added, dissolved at room temperature, and then cooled to 5 ° C. A solution prepared by dissolving 50 g (370.4 mmol) of 2,2,2-trifluoroethylamine hydrochloride in 60 g of water was added dropwise thereto at 5 ° C. After 85 g of tert-butyl methyl ether was added and stirred for 30 minutes, a solution of 43.9 g (388.9 mmol) of chloroacetyl chloride dissolved in 15 g of tert-butyl methyl ether was added dropwise while keeping at 5 ° C. The reaction solution was heated to 10 ° C. and stirred for 1 hour. After disappearance of 2,2,2-trifluoroethylamine was confirmed by gas chromatography, the temperature was raised to room temperature and liquid separation was carried out. The aqueous layer was extracted with 100 g of tert-butyl methyl ether, the organic layers were combined, the solvent was distilled off under reduced pressure to adjust the liquid volume, and 2-chloro-N- (2,2,2-trifluoroethyl) acetamide Of a tert-butyl methyl ether solution was obtained. As a result of the internal standardization by high performance liquid chromatography, the yield was 65 g, the yield was 100%, and the concentration was 25.1 wt%. The melting point of 2-chloro-N- (2,2,2-trifluoroethyl) acetamide taken out by dropping the obtained solution in heptane and crystallizing it was 53.8 ° C. (DSC).
References:
JP5652628,2015,B2 Location in patent:Paragraph 0057
753-90-2
329 suppliers
$24.00/1g

79-04-9
382 suppliers
$12.00/5g

170655-44-4
74 suppliers
$38.00/100mg