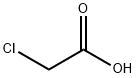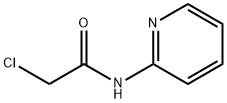
2-CHLORO-N-PYRIDIN-2-YL-ACETAMIDE synthesis
- Product Name:2-CHLORO-N-PYRIDIN-2-YL-ACETAMIDE
- CAS Number:5221-37-4
- Molecular formula:C7H7ClN2O
- Molecular Weight:170.6
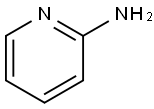
504-29-0

79-04-9

5221-37-4
1. Dissolve 2-aminopyridine (2.8 g, 30 mmol) in 50 mL of ethylene dichloride (25 mL) in a 50 mL glass container. 2. Slowly add chloroacetyl chloride dropwise to the above solution. 3. seal the glass vessel with a TFM Teflon lid and place it in the rotor of the microwave reactor. 4. Microwave irradiate the mixture for 5 minutes at 300 W power and 80 °C. 5. 5. Upon completion of the reaction, the pH of the reaction mixture was adjusted to 9 with saturated aqueous sodium hydroxide solution. 6. The reaction mixture was extracted twice with dichloroethane. 7. The organic layers were combined and dried with anhydrous sodium sulfate. 8. The solvent was removed by rotary evaporator to give the crude product. 9. The crude product was recrystallized using acetonitrile to give 4.9 g (97% yield) of 2-chloro-N-pyridin-2-ylacetamide as a pink solid. 10. The melting point of the product is 110-115 °C. 11. The product was analyzed by IR (Kjeldahl). 11. The product was analyzed by IR (KBr) and showed characteristic absorption peaks at 3443, 3226, 1683, 1581, 1330, 1198, 775 cm-1. 12. 1H NMR (KBr) analysis showed the product to be in the range of 1H NMR (1H NMR, 1H NMR). 12. 1H NMR (CDCl3) data: δ 4.2 (2H, s), 7.1 (1H, d), 7.7 (1H, t), 8.2 (1H, d, J = 8.3 Hz), 8.4 (1H, d, J = 4.9 Hz), 8.95 (1H, bs). 13. 13C NMR (CDCl3) data: δ 43.2, 111.4, 121.0, 139.1, 148.2, 150.7, 164.9. 14. EIMS analysis showed a molecular ion peak m/z of 170.6 (M+).
Yield:5221-37-4 97%
Reaction Conditions:
Stage #1: 2-aminopyridine;chloroacetyl chloride in 1,2-dichloro-ethane at 80; for 0.0833333 h;Microwave irradiation;
Stage #2: with sodium hydroxide in water;1,2-dichloro-ethane;
Steps:
1.1
2-aminopyridine (2.8 g, 30 mmol) was dissolved in dichloroethane (25 ml) in a 50 ml-glass vessel and chloroacetyl chloride was added dropwise thereto. The glass vessel was capped with a TFM teflon cover and placed in a rotor in a microwave reactor. The mixture was irradiated by microwave at 300 W and 80° C., for 5 min. After irradiating for 5 min., the reaction mixture was adjusted to pH9 with a saturated aqueous solution of sodium hydroxide and extracted twice with dichloroethane. The organic layer was dried over anhydrous sodium sulfate. The solvent was removed by using a rotary evaporator and the desired pink solid product of 4.9 g (97%) was obtained by recrystallization with acetonitrile. mp 110-115° C.; IR (KBr) 3443, 3226, 1683, 1581, 1330, 1198, 775 cm-1; 1H NMR (CDCl3) 4.2 (2H, s), 7.1 (1H, d), 7.7 (1H, t), 8.2 (1H, d, J=8.3 Hz), 8.4 (1H, d, J=4.9 Hz), 8.95 (1H, bs); 13C NMR (CDCl3) 43.2, 111.4, 121.0, 139.1, 148.2, 150.7, 164.9; EIMS m/z 170.6 (M+)
References:
US2007/36715,2007,A1 Location in patent:Page/Page column 4-5

94258-23-8
1 suppliers
inquiry

5221-37-4
81 suppliers
$37.00/250mg