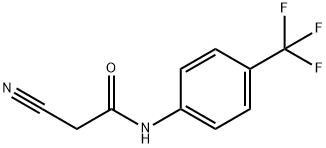
2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide synthesis
- Product Name:2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide
- CAS Number:24522-30-3
- Molecular formula:C10H7F3N2O
- Molecular Weight:228.17
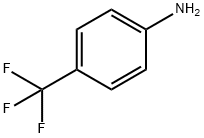
455-14-1
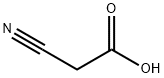
372-09-8
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
In 700 L of tetrahydrofuran, 63.4 kg of cyanoacetic acid was dissolved and stirred at room temperature and under nitrogen protection. The resulting solution was cooled to 0 to 10°C, followed by slow dropwise addition of N-methylmorpholine at the same temperature over about 1 hour. Next, 100.0 kg of 4-trifluoromethylaniline was added dropwise. Continuing at the same temperature, 91.3 kg of isopropyl chlorocarbonate was slowly added dropwise to the reaction mixture over about 1 hour. After the dropwise addition was completed, the reaction was continued with stirring for 1 to 2 hours. After completion of the reaction, 200 L of water was added to the reaction mixture, stirred and left to stratify. The organic layer (upper layer) was washed with 16.7% brine, followed by the addition of 400 L of isopropanol and concentrated under reduced pressure until the volume of liquid was reduced to 400 L. 400 L of isopropanol was added again, and the concentration was repeated under reduced pressure until the volume of liquid was 400 L. 100 L of isopropanol was added to the concentrated solution at 20 to 30 °C followed by a slow dropwise addition of 500 L of water at about 20 °C. The reaction was completed with the addition of 500 L of water. After completion of the dropwise addition, stirring was continued for 1 hour at the same temperature. The mixture was cooled to 0 to 10°C and stirred at that temperature for 1 hour. The precipitated crystals were collected by filtration and dried to give 134.5 kg of 2-cyano-N-(4-(trifluoromethyl)phenyl)acetamide (IV) in 95.0% yield.

455-14-1
522 suppliers
$5.00/10g

372-09-8
368 suppliers
$20.00/25g
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
142 suppliers
$21.00/250mg
Yield: 95%
Reaction Conditions:
Stage #1:cyanoacetic acid with 4-methyl-morpholine in tetrahydrofuran at 0 - 10; for 1 h;Industry scale;
Stage #2:4-trifluoromethylphenylamine with isopropyl chloroformate in tetrahydrofuran at 0 - 10; for 2 - 3 h;Product distribution / selectivity;Industry scale;
Steps:
1; 1 Preparation of 4-trifluoromethyl cyanoacetoanilide (compound (IV))
Example 1 Preparation of 4-trifluoromethyl cyanoacetoanilide (compound (IV)) In 700 L of tetrahydrofuran, 63.4 kg of cyanoacetic acid was dissolved with stirring in nitrogen atmosphere at room temperature. This solution was cooled to 0 to 10°C, and then N-methylmorpholine was added dropwise with stirring at the same temperature in about an hour. Then, 100.0 kg of 4-trifluoromethylaniline was added dropwise. To this reaction mixture, 91.3kg of isopropyl chlorocarbonate was added dropwise with stirring at the same temperature in about an hour. Further, the stirring was continued for 1 to 2 hours. After the reaction was finished, 200 L of water was added to the reaction mixture, which was stirred and then allowed to stand for separation. An organic layer (upper layer) was washed with 16.7 % brine, and then 400 L of isopropyl alcohol was added thereto, which was concentrated under reduced pressure until the liquid amount became 400 L. To the condensed solution, 400 L of isopropyl alcohol was added, which was concentrated again under reduced pressure until the liquid amount became 400 L. To the condensed solution, 100 L of isopropyl alcohol was added at 20 to 30°C with stirring, and then 500 L of water was added dropwise in about an hour. Then, the stirring was further continued for an hour at the same temperature. This mixture was cooled to 0 to 10°C with stirring, and then stirred at the same temperature for an hour. Precipitated crystals were collected by filtration and dried, to give 134.5 kg of the title compound (IV) in a yield of 95.0 %.
References:
Astellas Pharma Inc. EP1609778, 2005, A1 Location in patent:Page/Page column 5; 8-9

455-14-1
522 suppliers
$5.00/10g
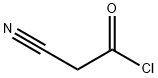
16130-58-8
19 suppliers
inquiry
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
142 suppliers
$21.00/250mg

455-14-1
522 suppliers
$5.00/10g
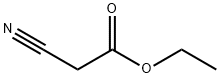
105-56-6
461 suppliers
$10.00/5ml
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
142 suppliers
$21.00/250mg

455-14-1
522 suppliers
$5.00/10g
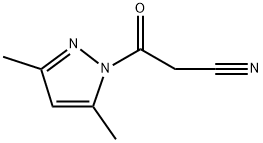
36140-83-7
103 suppliers
$23.00/250mg
![2-cyano-N-[4-(trifluoromethyl)phenyl]acetamide](/CAS/GIF/24522-30-3.gif)
24522-30-3
142 suppliers
$21.00/250mg