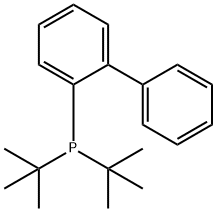
2-(Di-tert-butylphosphino)biphenyl synthesis
- Product Name:2-(Di-tert-butylphosphino)biphenyl
- CAS Number:224311-51-7
- Molecular formula:C20H27P
- Molecular Weight:298.4
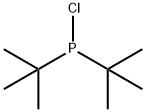
13716-10-4
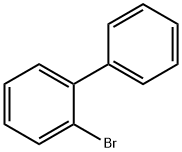
2052-07-5

224311-51-7
Grignard reagent was prepared by adding 40 g of 2-bromobiphenyl, 5 g of magnesium shavings and 400 ml of anhydrous THF to a 1 L three-necked flask under nitrogen protection and refluxed for 2 hours. After the reaction mixture was cooled to room temperature, 2 g of tetrakis (triphenylphosphine) palladium was added and stirred for 30 minutes. Subsequently, 33 g of di-tert-butylphosphonium chloride was slowly added dropwise at room temperature and the reaction mixture was refluxed for 2 hours after completion of the dropwise addition. Upon completion of the reaction, the mixture was quenched by slow dropwise addition to 200 mL of saturated aqueous ammonium chloride solution under cooling in an ice water bath. The organic phase was separated, dissolved and crystallized by adding methanol, and filtered to give 49 g of white solid 2-(di-tert-butylphosphino)biphenyl in 95.7% yield.

13716-10-4
283 suppliers
$22.00/1g

2052-07-5
397 suppliers
$16.30/1g

224311-51-7
299 suppliers
$6.00/250mg
Yield:224311-51-7 95.7%
Reaction Conditions:
Stage #1: 2-Bromobiphenylwith magnesium in tetrahydrofuran; for 2 h;Inert atmosphere;Reflux;
Stage #2: di(tert-butyl)chlorophosphinewith tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran at 20; for 2 h;Reflux;Inert atmosphere;Reagent/catalyst;
Steps:
2 Example 2. Preparation of 2- (di-t-butyl phosphino) biphenyl
Under nitrogen protection,1L three bottles,From 40 g of 2-bromobiphenyl,5 g of magnesium turnings and 400 ml of anhydrousTHF to produce Grignard reagent,Refluxed for 2 hours,Down to room temperature,2 g of tetrakis (triphenylphosphine) palladium was added,Stirred for 30 minutes,33 g of di-tert-butylphosphonium chloride was added dropwise at room temperature,The reaction was refluxed for 2 hours.And the mixture was added dropwise to the reaction solution under ice-water bath200 mL of saturated aqueous ammonium chloride was quenched,Liquid separation,The organic phase is dissolved,Add methanol crystallization,Filtration gave 49 g of white 2- (di-tert-butylphosphine) biphenyl, and the yield was 95.7%.
References:
CN105859774,2016,A Location in patent:Paragraph 0062; 0063; 0064; 0065; 0066; 0067; 0068; 0069

13716-10-4
283 suppliers
$22.00/1g
82214-69-5
25 suppliers
$255.00/50mL

224311-51-7
299 suppliers
$6.00/250mg
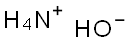
1336-21-6
740 suppliers
$14.56/250ML

2052-07-5
397 suppliers
$16.30/1g

224311-51-7
299 suppliers
$6.00/250mg
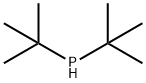
819-19-2
159 suppliers
$74.00/1g
![Methanesulfonic acid, 1,1,1-trifluoro-, [1,1'-biphenyl]-2-yl ester](/CAS/20200611/GIF/17763-65-4.gif)
17763-65-4
5 suppliers
inquiry

224311-51-7
299 suppliers
$6.00/250mg
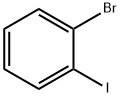
583-55-1
483 suppliers
$7.00/5g

224311-51-7
299 suppliers
$6.00/250mg