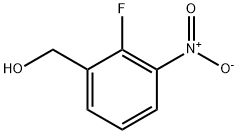
(2-Fluoro-3-nitrophenyl)methanol synthesis
- Product Name:(2-Fluoro-3-nitrophenyl)methanol
- CAS Number:946126-95-0
- Molecular formula:C7H6FNO3
- Molecular Weight:171.13
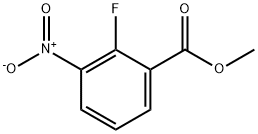
946126-94-9

946126-95-0
General procedure for the synthesis of 2-fluoro-3-nitrobenzyl alcohol from methyl 2-fluoro-3-nitrobenzoate: 1. DIBAL (115.7 mL, 1.0 M toluene solution) was slowly added to a toluene solution (92 mL) of methyl 2-fluoro-3-nitrobenzoate (9.22 g, 46.3 mmol) at -78 °C. 2. The reaction mixture was stirred at -78 °C for 30 min, then warmed to 0 °C and continued stirring for 30 min. 3. The reaction solution was cooled again to -78 °C and methanol, saturated Rochelle brine and ethyl acetate were added sequentially. 4. The mixture was transferred to room temperature, stirred for 1 hour and then extracted three times with ethyl acetate. 5. The organic layers were combined, washed with saturated brine and dried over anhydrous magnesium sulfate. 6. The organic phase was concentrated under pressure to give 2-fluoro-3-nitrobenzyl alcohol as a brown oil (7.52 g, 95% yield). 7. The product was analyzed by 1H NMR. 7. The product was confirmed by 1H NMR (CDCl3): δ 7.95 (m, 1H), 7.84 (t, 1H), 7.31 (t, 1H), 4.87 (s, 2H). 8. HPLC analysis: Rt = 7.52 min (conditions were the same as in the preparative embodiment of compound 3a-1).

946126-94-9
165 suppliers
$28.00/1g

946126-95-0
73 suppliers
$48.00/250mg
Yield:946126-95-0 95%
Reaction Conditions:
Stage #1: 2-fluoro-3-nitrobenzoic acid methyl esterwith diisobutylaluminium hydride in toluene at -78 - 0; for 1 h;
Stage #2: with water;rochelle salt in methanol;toluene at -78 - 20; for 1 h;
Steps:
2; 3
Compound 2c-1: (2-Fluoro-3-nitrophenyl)methanol; [Show Image] [Show Image] DIBAL (115.7 mL, 1.0 M in toluene) was added at -78°C to a solution of 2-fluoro-3-nitrobenzoic acid methyl ester (compound 2b-1) (9.22 g, 46.3 mmol) in toluene (92 mL), and the reaction mixture was stirred at -78°C for 30 minutes and at 0°C for 30 minutes. The resultant reaction solution was cooled again to -78°C, and methanol, aqueous saturated Rochelle salt solution and ethyl acetate were added thereto. The reaction mixture was then stirred at room temperature for 1 hour, and extracted three times with ethyl acetate. The organic layer was washed with saturated saline and dried over magnesium sulfate. The title compound (7.52 g, 95%) was then obtained by vacuum concentration as a brown oil. 1H NMR (CDCl3) δ (ppm): 7.95 (m, 1H), 7.84 (t, 1H), 7.31 (t, 1H), 4.87 (s, 2H). HPLC Rt = 7.52 min. HPLC conditions were the same as those for the manufacturing example for compound 3a-1.
References:
EP1982982,2008,A1 Location in patent:Page/Page column 198
317-46-4
367 suppliers
$6.00/1g

946126-95-0
73 suppliers
$48.00/250mg
53553-14-3
83 suppliers
$14.00/100mg

946126-95-0
73 suppliers
$48.00/250mg
3970-35-2
284 suppliers
$6.00/250mg

946126-95-0
73 suppliers
$48.00/250mg