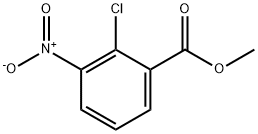
METHYL 2-CHLORO-3-NITROBENZOATE synthesis
- Product Name:METHYL 2-CHLORO-3-NITROBENZOATE
- CAS Number:53553-14-3
- Molecular formula:C8H6ClNO4
- Molecular Weight:215.59

67-56-1
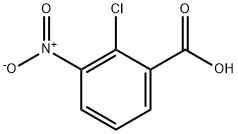
3970-35-2

53553-14-3
General procedure for the synthesis of methyl 2-chloro-3-nitrobenzoate from methanol and 2-chloro-3-nitrobenzoic acid: 1. to a solution of 2-chloro-3-nitrobenzoic acid (10.0 g, 49.6 mmol) in methanol (80 mL) was added concentrated sulfuric acid (2.0 mL). 2. The reaction mixture was stirred overnight under reflux conditions. 3. Upon completion of the reaction, the methanol was removed by vacuum concentration. 4. Water was added to the residue and extracted with ethyl acetate. 5. The organic phase was washed sequentially with water, saturated sodium bicarbonate solution and saturated brine. 6. 6. The organic phase was dried with magnesium sulfate. 7. The target compound, methyl 2-chloro-3-nitrobenzoate (10.6 g, 99% yield), was obtained as a white solid by vacuum concentration. 8. The product was characterized by 1H NMR (CDCl3) and HPLC: 1H NMR (CDCl3) δ (ppm): 7.95 (dd, 1H), 7.84 (dd, 1H), 7.48 (t, 1H), 3.98 (s, 3H). HPLC Rt = 11.88 min. 150 × 6.0 mm); mobile phase: 0-20 min, acetonitrile/water = 10/90 to 100/0 (gradient), 20-30 min, acetonitrile/water = 100/0 (isocratic); flow rate: 1 mL/min.

67-56-1
790 suppliers
$7.29/5ml-f

3970-35-2
284 suppliers
$6.00/250mg

53553-14-3
83 suppliers
$14.00/100mg
Yield: 100%
Reaction Conditions:
Stage #1:2-chloro-3-nitrobezoic acid with oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 6 h;
Stage #2:methanol in dichloromethane
Steps:
22.i
A suspension of 2-chloro-3-nitrobenzic acid (20 g, 99 mmol) in dichloromethane (800 inL) was cooled in an ice bath. Dimethylformamide (0.40 inL) was added to the reaction mixture followed by a dropwise addition of oxalyl chloride (13.85 g, 109 mmol). The reaction mixture was allowed to warm to room temperature and stirred for 6 h. Methanol(200 mL) was added dropwise to the reaction mixture and the reaction mixture was stirred overnight. The reaction mixture was concentrated in vacuo, and the residue was dissolved in dichloromethane and passed through a plug of silica eluting with a 50% ethyl acetate/n-hexanes mixture. The filtrate was concentrated in vacuo to give the title compound (21.5 g, 100%). 1H NMR (CDCl3) δ 3.98 (s, 3H), 7.48 (t, J = 7.8 Hz, IH), 7.84 (d, J = 8.2 Hz, IH), 7.95 (d, J = 7.8 Hz, IH).
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED WO2008/51533, 2008, A2 Location in patent:Page/Page column 95

3970-35-2
284 suppliers
$6.00/250mg

53553-14-3
83 suppliers
$14.00/100mg

67-56-1
790 suppliers
$7.29/5ml-f

79-37-8
490 suppliers
$17.67/10gm:

3970-35-2
284 suppliers
$6.00/250mg

53553-14-3
83 suppliers
$14.00/100mg

3970-35-2
284 suppliers
$6.00/250mg

18107-18-1
210 suppliers
$33.00/5g

53553-14-3
83 suppliers
$14.00/100mg

75-09-2
1264 suppliers
$10.00/25 mL

3970-35-2
284 suppliers
$6.00/250mg

53553-14-3
83 suppliers
$14.00/100mg