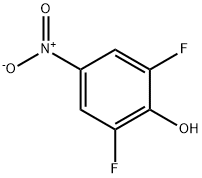
2,6-Difluoro-4-nitrophenol synthesis
- Product Name:2,6-Difluoro-4-nitrophenol
- CAS Number:658-07-1
- Molecular formula:C6H3F2NO3
- Molecular Weight:175.09
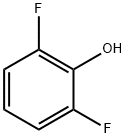
28177-48-2

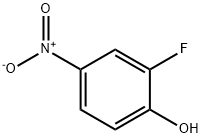
141-78-6

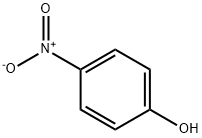
658-07-1
The general procedure for the synthesis of 2,6-difluoro-4-nitrophenol from 2,6-difluorophenol and ethyl acetate is as follows: with reference to Example 103, 2,6-difluorophenol (2.00 g) was dissolved in acetic acid (20 ml), and 60% nitric acid (1.20 ml) was slowly added dropwise under ice bath conditions. After the dropwise addition, the reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the mixture was poured into ice water and extracted with ethyl acetate. The extract was washed with saturated saline and the organic layer was dried with anhydrous magnesium sulfate. The dried organic layer was filtered and the filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography with the eluent being a mixed solvent with the volume ratio of hexane/ethyl acetate=3/1→2/1, resulting in the target compound 2,6-difluoro-4-nitrophenol (1.37 g, 51% yield) as a light yellow solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3), δppm: 7.95 (2H, m).

28177-48-2
313 suppliers
$15.00/5 g

141-78-6
683 suppliers
$20.50/250ml

658-07-1
116 suppliers
inquiry
Yield: 51%
Reaction Conditions:
with nitric acid in hexane;acetic acid
Steps:
R.103 2,6-Difluoro-4-nitrophenol
Reference Example 103 2,6-Difluoro-4-nitrophenol To a solution of 2,6-difluorophenol (2.00 g) in acetic acid (20 ml) was added dropwise 60% nitric acid (1.20 ml) in an ice bath and the mixture was stirred at room temperature for 1 hour. After pouring into iced water, the reaction mixture was extracted with ethyl acetate. The extract was washed with brine. The organic layer was dried over anhydrous magnesium sulfate, filtered and the filtrate concentrated in vacuo. The residue was purified by chromatography on a silica gel column using hexane/ethyl acetate=3/1→2/1 as an eluant to give the desired compound (1.37 g, yield 51%) as a pale yellow solid. 1H NMR (400 MHz, CDCl3) δ ppm: 7.95 (2H, m).
References:
Sankyo Company, Limited US6555556, 2003, B1

28177-48-2
313 suppliers
$15.00/5 g

658-07-1
116 suppliers
inquiry
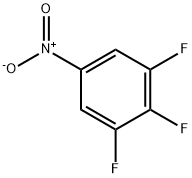
66684-58-0
216 suppliers
$10.00/1g

658-07-1
116 suppliers
inquiry
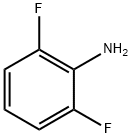
5509-65-9
497 suppliers
$10.00/10g

658-07-1
116 suppliers
inquiry