2-Hydroxy-3-cyanopyridine synthesis
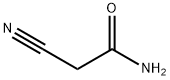
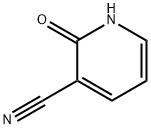
- Product Name:2-Hydroxy-3-cyanopyridine
- CAS Number:20577-27-9
- Molecular formula:C6H4N2O
- Molecular Weight:120.11
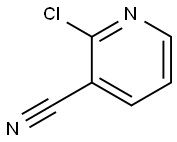
6602-54-6

20577-27-9
The general procedure for the synthesis of 2-oxo-1,2-dihydropyridine-3-carbonitrile from 2-chloro-3-cyanopyridine was as follows: acetic acid (826 mL) was slowly added to 2-chloro-3-cyanopyridine (250 g), and the mixture was subsequently heated to reflux and held for 48 hours. Upon completion of the reaction, the reaction mixture was quenched by careful pouring into an ice-water mixture. The resulting precipitate was collected by filtration, washed well with deionized water and dried under appropriate conditions to give the target product 2-hydroxy-3-cyanopyridine. The yield of this step was 95%. The structure of the product was confirmed by 1H NMR (300 MHz, DMSO-d6): δ 12.57 (s, 1H), 8.16 (dd, J = 3.6 Hz, 1H), 7.80 (dd, J = 3.6 Hz, 1H), 6.37 (t, 1H); the mass spectrum (ES) showed m/z of 121.

6602-54-6
512 suppliers
$6.00/10g

20577-27-9
169 suppliers
$14.00/1g
Yield:20577-27-9 100%
Reaction Conditions:
with acetic acid for 12 h;Reflux;
Steps:
2-Bromo-3-cyanopyridine(24).
2-Chloro-3-cyanopyridine 23 (3.68 mg, 26 mmol) was boiled underreflux with AcOH (100 mL) for 12 h. The evaporation residue, in THF (80 mL)and H2O (20 mL), was boiled under reflux for 4 h. Evaporation gave 3-cyanopyridin-2-one(3.12 g, quant.) as white crystals: mp 110-114°C (lit.13174-175°C); 1H NMR d 6.47 (1 H, t, J =6.6 Hz, 5-H), 7.91 (1 H, dd, J = 6.5, 2.1 Hz, 4-H), 8.14 (1 H, dd, J =7.0, 2.1 Hz, 6-H), 11.48 (1 H, s, 1-H); 13C NMR d 105.80 (3-C), 124.05(5-C), 141.87 (CN), 144.50 (6-H), 150.02 (4-C), 154.20 (2-C); MS m/z 143.0214(M + Na)+ (C6H4N2NaO requires143.0222). Bu4NBr (8.55 g, 26 mmol) and P2O5(3.67 g, 26 mmol) were heated in PhMe (250 mL) at 80°C for 30 min. The above3-cyanopyridin-2-one (1.59 g, 13 mmol) was added and the mixture was boiledunder reflux for 12 h. The mixture was cooled, poured into cold water andextracted (EtOAc, 2 ×). Drying and evaporation gave 24 (2.24 g, 94%) asoff-white crystals.
References:
Kumpan, Katerina;Nathubhai, Amit;Zhang, Chenlu;Wood, Pauline J.;Lloyd, Matthew D.;Thompson, Andrew S.;Haikarainen, Teemu;Lehti?, Lari;Threadgill, Michael D. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12299,p. 3013 - 3032] Location in patent:supporting information