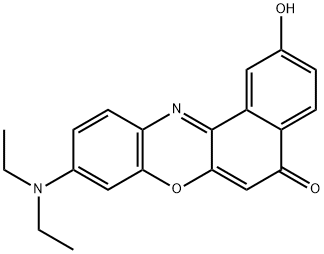
2-hydroxy nile red synthesis
- Product Name:2-hydroxy nile red
- CAS Number:188712-75-6
- Molecular formula:C20H18N2O3
- Molecular Weight:334.37
Yield:188712-75-6 92%
Reaction Conditions:
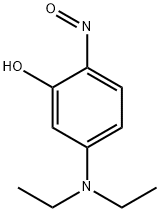
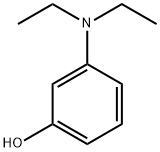
Stage #1: 3-diethylaminophenolwith hydrogenchloride;sodium nitrite in water at 0; for 2.66667 h;
Stage #2: 1,6-dihydroxynaphthalene in N,N-dimethyl-formamide; for 3 h;Reflux;
Steps:
13A; 14A EXAMPLE 14A
EXAMPLE 13A A solution containing 3-diethylaminophenol (15.0 g, 90.7 mmol) in concentrated HCl (100 mL) was prepared and cooled to 0° C. in an ice-bath. NaNO2 (6.9 g, 100.0 mmol) in water (50 mL) was added slowly over 40 minutes such that no brown NOx vapours were observed. The reaction was left to stir for 2 hours, after which the thick precipitate was filtered using a Büchner funnel and washed with small portions of water (3×50 mL). After drying the solid for 1 hour on the Büchner funnel, the solid material was dissolved into EtOH (70 mL), Et2O (35 mL) was added and the solution stored at -20° C. overnight to allow for crystallization. The next day, the solid material was collected by vacuum filtration, using a Büchner funnel and air dried. The product was an orange/red solid (8.8 g, 50%). EXAMPLE 14A The compound of Example 13A (7.0 g, 36.0 mmol) and 1,6-dihydroxynaphthelene (5.9 g, 36.1 mmol) were refluxed in DMF (100 mL) for 3 hours. After the solvents were removed under vacuum, the residue was redissolved in methanol and adsorbed onto silica and purified using silica gel column chromatography (MeOH:NEt3=50:1) to afford the title compound as a dark purple solid (11.1 g, 92%).
References:
US9829481,2017,B2 Location in patent:Page/Page column 15; 16
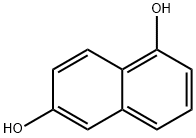
575-44-0
376 suppliers
$7.00/10g
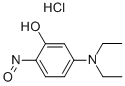
25953-06-4
39 suppliers
$145.00/500mg

188712-75-6
15 suppliers
inquiry
91-68-9
304 suppliers
$9.00/25g

188712-75-6
15 suppliers
inquiry