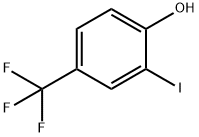
2-Iodo-4-(trifluoroMethyl)phenol synthesis
- Product Name:2-Iodo-4-(trifluoroMethyl)phenol
- CAS Number:463976-21-8
- Molecular formula:C7H4F3IO
- Molecular Weight:288.01
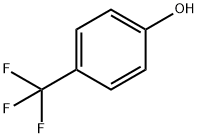
402-45-9

463976-21-8
S3. 4-(Trifluoromethyl)phenol (6) (241 mmol, 39 g) was dissolved in a solvent mixture of tetrahydrofuran (200 mL) and water (200 mL) under stirring conditions. Iodine (265 mmol, 67 g) and sodium carbonate (265 mmol, 28 g) were added sequentially. The reaction mixture was stirred at room temperature for 24 h. The progress of the reaction was monitored by TLC until 4-(trifluoromethyl)phenol (6) was fully reacted. Post-treatment step: 5% aqueous thiourea solution was added to quench unreacted iodine, followed by three extractions with ether. The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography with the eluent being a mixed solvent of petroleum ether and dichloromethane (the volume ratio was tapered from 10:1 to 6:1) to afford the target compound 2-iodo-4-(trifluoromethyl)phenol (7) (42.7 g) in 63% yield.

402-45-9
373 suppliers
$20.00/5 / G

463976-21-8
65 suppliers
$45.00/10mg
Yield: 75%
Reaction Conditions:
with iodine;sodium hydrogencarbonate;thiourea in tetrahydrofuran;water
Steps:
12.A 6-(5-Trifluoromethyl-3-methyl-benzofuran-2-suffonyl)-2H-pyridazin-3-one
Step A: a,a a-Trifluoro-o-iodo-p-cresol. A mixture of iodine (91.6 mmol, 23.2 g) and sodium bicarbonate (91.6 mmol, 7.7 g) was added to a solution of α,α,α-trifluoro-p-cresol (83.3 mmol, 13.5 g) in THF (90 mL) and H2O (90 mL) and the reaction mixture was allowed to stand at room temperature overnight. Sufficient thiourea (5% solution) was added to remove the excess iodine as indicated by the color change of the reaction from deep violet to brown. The reaction mixture was extracted with ether (3*100 mL), the extract was dried, filtered and the filtrate was concentrated to obtain a brown oil. This oil was distilled (bp 105° C. at 44 mm Hg) to obtain α,α,α-trifluoro-o-iodo-p-cresol (4.1 g, 75% pure, admixed with the starting α,α,α-trifluoro-p-cresol).
References:
Mylari, Banavara L. US2002/143017, 2002, A1
109-99-9
1101 suppliers
$9.00/5ml

402-45-9
373 suppliers
$20.00/5 / G

463976-21-8
65 suppliers
$45.00/10mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

463976-21-8
65 suppliers
$45.00/10mg