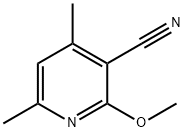
2-METHOXY-4,6-DIMETHYLNICOTINONITRILE synthesis
- Product Name:2-METHOXY-4,6-DIMETHYLNICOTINONITRILE
- CAS Number:65515-39-1
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
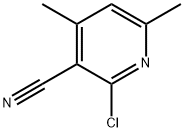
14237-71-9

124-41-4

65515-39-1
General procedure for the synthesis of 2-methoxy-4,6-dimethylnicotinonitrile from 2-chloro-3-cyano-4,6-dimethylpyridine and sodium methanol: A methanolic solution of sodium methanol (30 mass %, 175 mL, 940 mmol) was added slowly and dropwise to a methanolic solution (450 mL) of 2-chloro-4,6-dimethylpyridine-3-carbonitrile (75 g, 441.1 mmol) The reaction was carried out in a water bath. Subsequently, a methanolic solution of sodium methanolate (25 mass %, 250 mL, 1090 mmol) was added dropwise. The reaction mixture was stirred for 1 hour and then poured into ice water and continued to stir for 30 minutes and the solid product was collected by filtration. The filter cake was washed with hexane and dried in a vacuum oven overnight. The aqueous phase filtrate was extracted with dichloromethane and the organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The solid obtained by filtration was combined with the concentrated residue to give 2-methoxy-4,6-dimethylnicotinonitrile as a solid product (71.8 g, quantitative yield). Electrospray mass spectrometry (ES/MS) showed m/z: 163 ([M+H]+).

14237-71-9
191 suppliers
$6.00/250mg

124-41-4
730 suppliers
$12.00/25g

65515-39-1
72 suppliers
inquiry
Yield:65515-39-1 100%
Reaction Conditions:
in methanol; for 1 h;
Steps:
16 Preparation 162-methoxy-4,6-dimethyl-pyridine-3 -carbonitrile
Add a solution of NaOCH3 in MeOH (30 mass%, 175 mL, 940 mmol) drop wise to a solution of 2-chloro-4,6-dimethyl-pyridine-3 -carbonitrile (75 g, 441.1 mmol) inMeOH (450 mL) in a water bath followed by another drop wise addition of NaOCH3 in MeOH (25 mass%, 250 mL, 1090 mmol). Stir the resulting mixture for 1 hr, pour in ice cold water, stir for 30 mm, and filter the resulting solid. Wash filter cake with hexane and dry in a vacuum oven overnight. Extract the aqueous filtrate with DCM, dry the organic phase over Mg504, filter and concentrate in vacuo. Combine the filtered solid and theevaporated residue to afford title compound as a solid (71.8 g, quantitative yield). ES/MS (mlz): 163 (M+H).
References:
WO2017/35060,2017,A1 Location in patent:Page/Page column 48; 49

67-56-1
790 suppliers
$9.00/25ml

14237-71-9
191 suppliers
$6.00/250mg

65515-39-1
72 suppliers
inquiry

14237-71-9
191 suppliers
$6.00/250mg

65515-39-1
72 suppliers
inquiry
769-28-8
255 suppliers
$5.00/1g

65515-39-1
72 suppliers
inquiry
