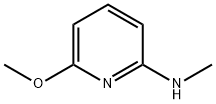
2-Methoxy-6-(methylamino)pyridine synthesis
- Product Name:2-Methoxy-6-(methylamino)pyridine
- CAS Number:88569-83-9
- Molecular formula:C7H10N2O
- Molecular Weight:138.17
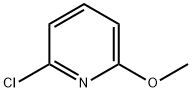
17228-64-7
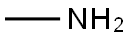
74-89-5

88569-83-9
General procedure for the synthesis of 2-methoxy-6-(methylamino)pyridine from 2-chloro-6-methoxypyridine and monomethylamine: 2-chloro-6-methoxypyridine (12.0 g, 85.6 mmol) was mixed with 40% aqueous methanamine (24 mL) in a sealed tube and reacted for 7 hr at 170 °C. After completion of the reaction, it was cooled to room temperature and the reaction mixture was diluted with 50 mL of water and subsequently extracted with dichloromethane (3 x 50 mL). The organic layers were combined, washed sequentially with 100 mL of water and 100 mL of brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (100-200 mesh, eluent 0 to 5% dichloromethane solution in methanol) to afford 6-methoxy-N-methylpyridin-2-amine (2.7 g, 31% yield) as a light yellow oil.1H NMR (400 MHz, CDCl3) δ 7.37 (t, J = 8.0 Hz, 1H), 6.04 (d, J = 8.0 Hz, 1H), 5.94 (d, J = 7.8 Hz, 1H), 4.39 (br. s, 1H), 3.86 (s, 3H), 2.90 (s, 3H).

17228-64-7
316 suppliers
$6.00/5g
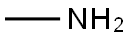
74-89-5
0 suppliers
$13.44/25ML

88569-83-9
179 suppliers
$20.00/1g
Yield: 31%
Reaction Conditions:
in water at 170; for 7 h;Sealed tube;
Steps:
3.1 Step 1: 6-methoxy-N-methylpyridin-2-amine
A mixture of 2-chloro-6-methoxypyridine (12.0 g, 85.6 mmol) and aqueous methanamine (40%, 24 mL) was stuffed at 170 °C in sealed tube for 7 h. After cooled, themixture was diluted with water (50 mL) and then extracted with dichloromethane (3 x 50 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, 100-200 mesh, 0 to 5% methanol in dichloromethane) to afford 6-methoxy-N-methyl-pyridin-2-amine (2.7 g, 31%) as a lightyellow oil. ‘H NMR (400 MHz, CDC13) 5 7.37 (t, J = 8.0 Hz, 1H), 6.04 (d, J = 8.0 Hz, 1H),5.94 (d, J = 7.8 Hz, 1H), 4.39 (br. s, 1H), 3.86 (s, 3H), 2.90 (s, 3H).
References:
F. HOFFMANN-LA ROCHE AG;GENENTECH, INC.;PATEL, Snahel WO2018/73193, 2018, A1 Location in patent:Page/Page column 49