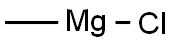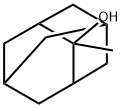
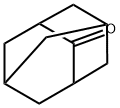
2-Methyl-2-adamantanol synthesis
- Product Name:2-Methyl-2-adamantanol
- CAS Number:702-98-7
- Molecular formula:C11H18O
- Molecular Weight:166.26
700-58-3
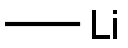
917-54-4

702-98-7
Dissolve 2-adamantanone (1.50 g, 10 mmol) in ether under nitrogen protection and cool to 0°C. Methyl lithium solution (7.5 mL, 1.6 M in ether, 12 mmol) was added slowly and dropwise. The reaction mixture was continued to be stirred at 0 °C for 1 h, then brought to room temperature and stirred overnight. Upon completion of the reaction, the reaction was quenched by the addition of saturated ammonium chloride solution and extracted three times with ether. The organic phases were combined, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by fast column chromatography (eluent: dichloromethane to 5% methanol/dichloromethane) to afford 2-methyl-2-adamantanol (56) as a white solid (1.58 g, 95% yield).1H-NMR (360 MHz, CDCl3) δ 2.20-2.16 (m, 2H), 1.89-1.75 (m, 6H), 1.68 ( br s, 4H), 1.57-1.54 (m, 2H), 1.48 (br s, 1H), 1.35 (s, 3H); 13C-NMR (90 MHz, CDCl3) δ 74.06, 39.30, 38.49, 35.33, 33.16, 27.73, 27.60, 27.22; ESI-MS: C11H18O(M+H)+ calculated value 167.3, measured value 167.3.
Yield:702-98-7 99%
Reaction Conditions:
Stage #1: 2-Adamantanone;methylmagnesium chloride in tetrahydrofuran at 0; for 0.5 h;
Stage #2: with ammonium chloride in tetrahydrofuran;
Steps:
3M Methylmagnesium chloride in THF (44 mL), was added through a canula to adamantan-2-one(10 g, 66.66 mmol) in THF (50 mL) at 0 °C. After stirring at 0 °C for 0.5 h, the reaction mixture was quenched by adding saturated NH4C1 solution. The organic layer was separated and the aqueous layer was extracted with EtOAc. The combined organic layer was washed with water and brine, dried over Na2S04 and the solvent was removed under reduced pressure to afford 2-methyladamantan-2-ol (11 g, 99% yield) as an off-white solid.
References:
WO2011/58582,2011,A1 Location in patent:Page/Page column 27
![Tricyclo[4.4.0.03,8]decan-2-ol, 2-methyl- (8CI)](/CAS/20210305/GIF/20497-75-0.gif)
20497-75-0
0 suppliers
inquiry

702-98-7
251 suppliers
$6.00/1g