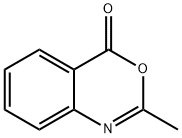
2-Methyl-4H-3,1-benzoxazin-4-one synthesis
- Product Name:2-Methyl-4H-3,1-benzoxazin-4-one
- CAS Number:525-76-8
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
Yield:525-76-8 100%
Reaction Conditions:
at 130; for 3 h;
Steps:
14
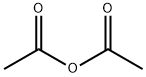
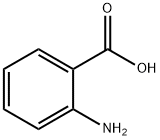
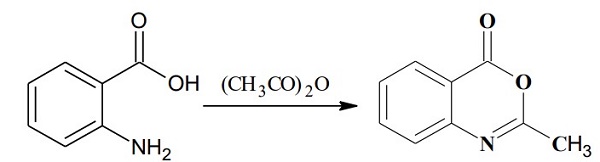
Synthesis of 2-methyl-4H-3,1-benzoxazin-4-one. Anthranilic acid (1.8 g, 13.1 mmol) and acetic anhydride (20 ml_, excess) were mixed and refluxed at 130 0C for 3 hours. The excess acetic anhydride was removed under high vacuum to afford 2-methyl-4H-3,1-benzoxazin-4-one (2.1 g, 100%) as an oily product: TLC (ethyl acetate / hexane, 1/2 v/v) Rf = 0.3.
References:
XENON PHARMACEUTICALS INC. WO2008/94909, 2008, A2 Location in patent:Page/Page column 99
201230-82-2
1 suppliers
inquiry
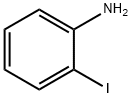
615-43-0
428 suppliers
$5.00/5g
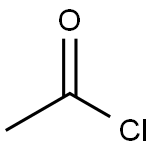
75-36-5
589 suppliers
$17.92/100G

525-76-8
142 suppliers
$51.79/10g
201230-82-2
1 suppliers
inquiry
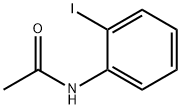
19591-17-4
57 suppliers
$55.00/100mg

525-76-8
142 suppliers
$51.79/10g
89-52-1
0 suppliers
$14.14/1gm:

525-76-8
142 suppliers
$51.79/10g