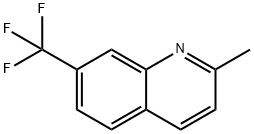
2-Methyl-7-(trifluoroMethyl)quinoline synthesis
- Product Name:2-Methyl-7-(trifluoroMethyl)quinoline
- CAS Number:324-32-3
- Molecular formula:C11H8F3N
- Molecular Weight:211.18

123-73-9
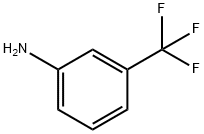
98-16-8

324-32-3
General procedure for the synthesis of 2-methyl-7-(trifluoromethyl)quinoline from trans-2-butenal and m-aminobenzotrifluoride: To an aqueous 6N HCl solution (50 mL) of 3-(trifluoromethyl)aniline (12.43 mL, 99.92 mmol) was slowly added (E)-but-2-enal (18.77 mL, 229.8 mmol) dropwise under reflux conditions. The reaction mixture was stirred at reflux for 3 hours. After completion of the reaction, it was cooled to room temperature and diluted by adding ethyl acetate (200 mL). The aqueous layer was separated and the pH of the aqueous layer was adjusted to about 9 with ammonium hydroxide, followed by extraction with dichloromethane (2 x 200 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel fast column chromatography (eluent ratio 5:1 hexane/ethyl acetate) to afford the target product 2-methyl-7-(trifluoromethyl)quinoline (6.1 g, 28.9% yield) as a solid.
123-42-2
594 suppliers
$22.50/100ml
109466-88-8
17 suppliers
inquiry

324-32-3
43 suppliers
$52.00/100mg
Yield:324-32-3 81%
Reaction Conditions:
with chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I);sodium t-butanolate in toluene at 70;Inert atmosphere;Schlenk technique;chemoselective reaction;
References:
Zhang, Song-Lin;Deng, Zhu-Qin [Organic and Biomolecular Chemistry,2016,vol. 14,# 38,p. 8966 - 8970] Location in patent:supporting information

98-16-8
390 suppliers
$9.00/5g

324-32-3
43 suppliers
$52.00/100mg
186602-93-7
27 suppliers
inquiry

324-32-3
43 suppliers
$52.00/100mg