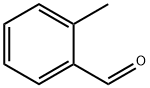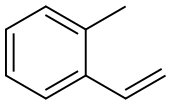
2-METHYLSTYRENE synthesis
- Product Name:2-METHYLSTYRENE
- CAS Number:611-15-4
- Molecular formula:C9H10
- Molecular Weight:118.18
Yield:611-15-4 96%
Reaction Conditions:
in tetradecyl(trihexyl)phosphonium decanoate;hexane for 2 h;
Steps:
9
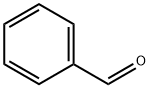
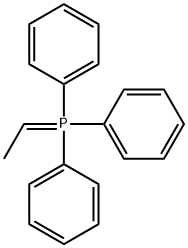
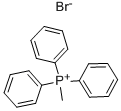
9. Wittig Reactions: Formation of methvlstyrene in phosphonium-based ionic liquids; [00064] To a cold (-78 °C) sample of trihexyl(tetradecyl) phosphoniumdecanoate (5 mL), 1 M phenylmagnesium bromide in THF (1.2 mL, 1.2 mmol) was added and allowed to slowly warm to room temperature. The THF was removed under vacuum, and to the resulting solution hexanes (approximately 2 mL) was added to reduce viscosity. Triphenylethylphosphonium bromide (0.4 g, 1.10 mmol) was then added to the solution. A color change from white to reddish orange was observed and the mixture was stirred under nitrogen for 1 hour. P{ H} NMR showed a distintive peak at 15.3 ppm for the deprotonation of triphenylethylphosphonium bromide to give the phosphorane. Benzaldehyde (0.11 g, 1.10 mmol) was then added to the mixture and an instant colour change from yellow to white was observed. The mixture was allowed to stir for 2 hours and then quenched with water. The product was extracted with dichloromethane which was dried using anhydrous magnesium sulphate to give methylstyrene (96%) analyzed by GC-MS. The presence of PI13PO was confirmed by mass spectrometry.
References:
SIMON FRASER UNIVERSITY WO2006/7703, 2006, A1 Location in patent:Page/Page column 24
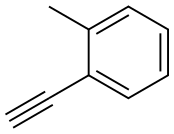
766-47-2
110 suppliers
$11.00/250mg

611-15-4
124 suppliers
$128.00/5g
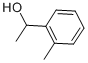
7287-82-3
79 suppliers
$49.89/5g

611-15-4
124 suppliers
$128.00/5g
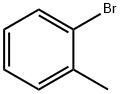
95-46-5
297 suppliers
$10.00/10 g
1826-67-1
208 suppliers
$51.29/100ml

611-15-4
124 suppliers
$128.00/5g