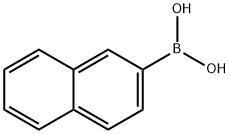
2-Naphthaleneboronic acid synthesis
- Product Name:2-Naphthaleneboronic acid
- CAS Number:32316-92-0
- Molecular formula:C10H9BO2
- Molecular Weight:171.99
150-46-9
200 suppliers
$14.00/25mL
580-13-2
506 suppliers
$6.00/1g

32316-92-0
476 suppliers
$6.00/1g
Yield:32316-92-0 90%
Reaction Conditions:
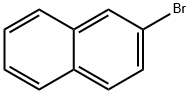
Stage #1: 2-bromonaphthalenewith n-butyllithium in tetrahydrofuran at -78; for 1 h;
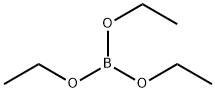
Stage #2: triethyl borate in tetrahydrofuran; for 6 h;
Steps:
1 Preparation of Compound C
A 500 mL 3-necked flask, place the 2-bromo-naphthalene 15 g (0.072 mol) and THF.It was cooled to -78°C and then, put the 2.5 Mn-BuLi 43.46 ml (0.109 mol) 1 hoursWhile stirring, and then insert the triethyl borate 18.5 ml (0.109 mol) was stirred for 6 hours. A 2M HCl to stop the reaction and extracted into CH2Cl2 and put enough water. Then, the compound to remove water from the solution C, and dried (11.22 g, yield: 90%) was obtained to, the structureIt was confirmed by H1-NMR.
References:
KR2015/22270,2015,A Location in patent:Paragraph 0266; 0267; 0274; 0275

580-13-2
506 suppliers
$6.00/1g

32316-92-0
476 suppliers
$6.00/1g

121-43-7
381 suppliers
$14.00/25mL

580-13-2
506 suppliers
$6.00/1g

32316-92-0
476 suppliers
$6.00/1g

150-46-9
200 suppliers
$14.00/25mL

580-13-2
506 suppliers
$6.00/1g

7732-18-5
507 suppliers
$13.50/100ML

32316-92-0
476 suppliers
$6.00/1g
