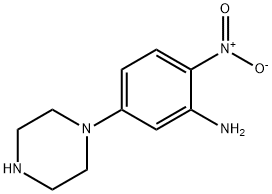
2-Nitro-5-(1-piperazinyl)aniline synthesis
- Product Name:2-Nitro-5-(1-piperazinyl)aniline
- CAS Number:96103-52-5
- Molecular formula:C10H14N4O2
- Molecular Weight:222.24

110-85-0
588 suppliers
$5.00/5G
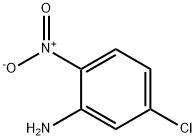
1635-61-6
300 suppliers
$13.00/25g

96103-52-5
24 suppliers
$105.00/1g
Yield:96103-52-5 84%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl acetamide at 120; for 21 h;
Steps:
1
5- (Piperazin-1'-vl)-2-nitroaniline (13) A mixture of 5-chloro-2-nitroaniline (5. 10 g, 29. 5 mmol), piperazine (12.6 g, 147 mmol) and anhydrous potassium carbonate (4.10 g. 29.7 mmol) in anhydrous N, N- dimethylacetamide (50 ml) was stirred at-120° for 21 h. After cooling to room temperature, the red-brown mixture was poured into crushed ice. The resultant orange suspension was extracted with dichloromethane (x 5). The combined organic extract was re-extracted into aqueous hydrochloric acid (x 2,5%). The combined orange aqueous phase was washed with dichloromethane and collected. Aqueous sodium hydroxide (30%) was added at 0° until pH- 10. A yellow precipitate started to form. The suspension was stirred at 0° for 45 min, and then left to stand at room temperature over the weekend before filtration. The crystalline yellow solid residue was washed with water, then with ether, and dried at the water pump. This was followed by further drying under vacuum in a dessicator for 20 h to give 5-(piperazin-1'-yl)-2-nitroaniline (13) (5.43 g, 84%) m. p. 166-9° (lit. 177- 8°, lit. 170°). 1H nmr (300 MHz, d4-MeOH/dl-TFA): 5 3.33 (m, 4H) H3', H5'; 3.56 (m, 4H) H2', H6' ; 6.27 (d, J2. 7 Hz, 1H) H6; 6.39 (dd, J9. 8,2. 7 Hz, 1H) H4; 7.97 (d, J9. 8 Hz, 1H) H3.
References:
WO2005/82894,2005,A1 Location in patent:Page/Page column 43
193902-98-6
12 suppliers
inquiry

96103-52-5
24 suppliers
$105.00/1g

1635-61-6
300 suppliers
$13.00/25g

96103-52-5
24 suppliers
$105.00/1g
2369-11-1
222 suppliers
$6.00/5g

96103-52-5
24 suppliers
$105.00/1g
![Acetamide, N-[4-(3-amino-4-nitrophenoxy)phenyl]-](/CAS/20210305/GIF/29178-59-4.gif)