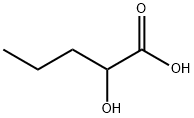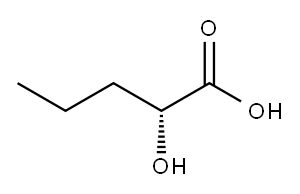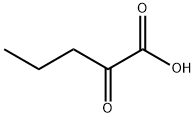
2-Oxopentanoic acid synthesis
- Product Name:2-Oxopentanoic acid
- CAS Number:1821-02-9
- Molecular formula:C5H8O3
- Molecular Weight:116.12
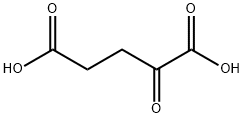
328-50-7
634 suppliers
$6.00/25g
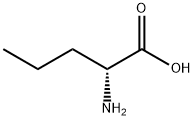
2013-12-9
309 suppliers
$5.00/1g
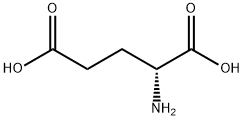
6893-26-1
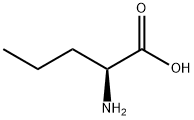
480 suppliers
$6.00/10g

1821-02-9
80 suppliers
$60.00/5mg
Yield:-
Reaction Conditions:
with pyridoxal 5'-phosphate;recombinant Lactobacillus salivarius UCC118 D-amino acid aminotransferase in aq. phosphate buffer; pH=7.5 at 30; for 0.0166667 h;Enzymatic reaction;
Steps:
General procedure: Amino donor specificity was determined using UPLC by measuring the rate of D-glutamate formation from α-ketoglutarate in the presence of various D-amino acids. The standard reaction mixture (1 ml) contained 100 mM potassium phosphate buffer (pH 7.5), 50 mM D-amino acid, 20 mM α-ketoglutarate, 0.05 mM pyridoxal-5′-phosphate (PLP) and the purified D-AAT. The enzyme reaction was run at 30 °C for 1 min and was stopped by adding 1 ml of 20% trichloroacetic acid. The reaction mixture was then incubated for 1 min on ice and 0.5 ml of 4 M NaOH was added to neutralize the mixture. UPLC analysis was performed using an ACQUITY UPLC TUV system consisting of a Waters Binary Solvent Manager, Sample Manager, FLR Detector and AccQ-Tag Ultra 2.1 × 100-mm column (Waters, Tokyo, Japan) with an eluent flow rate of 0.25 ml/min [14]. The column temperature was 30 °C, and the fluorescent wavelengths used for the FLR Detector were 350 and 450 nm. The eluent was linearly graduated using 85% 50 mM sodium acetate buffer (pH 5.9) and 15% acetonitrile.
References:
Kobayashi, Jyumpei;Shimizu, Yasuhiro;Mutaguchi, Yuta;Doi, Katsumi;Ohshima, Toshihisa [Journal of Molecular Catalysis B: Enzymatic,2013,vol. 94,p. 15 - 22]
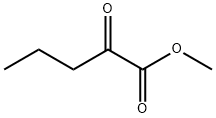
760-78-1
315 suppliers
$5.00/5g

1821-02-9
80 suppliers
$60.00/5mg
6376-59-6
42 suppliers
$155.00/500mg

1821-02-9
80 suppliers
$60.00/5mg
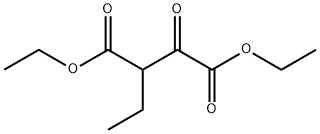
26103-77-5
1 suppliers
inquiry

1821-02-9
80 suppliers
$60.00/5mg