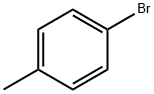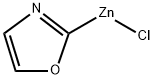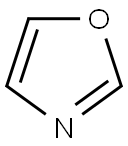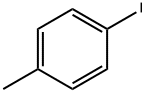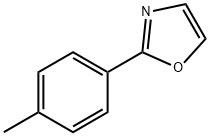
2-p-tolyloxazole synthesis
- Product Name:2-p-tolyloxazole
- CAS Number:62882-04-6
- Molecular formula:C10H9NO
- Molecular Weight:159.18
Yield:62882-04-6 51%
Reaction Conditions:
with [(2-di-tert-butylphosphino-2′,4′,6′-triisopropyl-1, 1′-biphenyl)-2-(2′-amino-1,1′-biphenyl)] palladium(II) methanesulfonate in tetrahydrofuran;2-methyltetrahydrofuran at 65; for 18 h;Inert atmosphere;regioselective reaction;Negishi Coupling;
Steps:
(15) Typical Procedure for the Synthesis of tert-Butyl 2-Methyl-2-{5-methyl-6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl}propanoate (2)
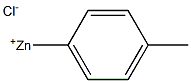
General procedure: A stock solution was prepared by addition of i-PrMgCl (60 mL,120 mmol) to oxazole (7.3 mL, 112 mmol) in THF (120 mL) at-15 °C, followed by addition of a solution of ZnCl2 (30.5 g, 224mmol) in MeTHF (105 mL) and warming to 22 °C. To a reaction vial, under nitrogen, was added the aryl halide 1 (2.50 mmol),followed by the oxazole zincate solution (20 mL, 7.50 mmol, 3.0equiv) and t-BuXPhos Pd G3 (105 mg, 5 mol%). The vial waswarmed to 65 °C for 18 hours under nitrogen. The reaction wasthen cooled and quenched by addition of 1 M HCl (20 mL). Compound2 was obtained by filtration.
References:
Calimsiz, Selcuk;Geier, Michael J.;Humphreys, Luke D.;Scott, Mark E.;Wang, Xiaotian [Synlett,2019,vol. 30,# 15,p. 1776 - 1781]

10200-70-1
0 suppliers
inquiry

62882-04-6
16 suppliers
inquiry