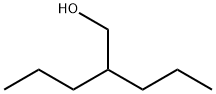
2-PROPYL-1-PENTANOL synthesis
- Product Name:2-PROPYL-1-PENTANOL
- CAS Number:58175-57-8
- Molecular formula:C8H18O
- Molecular Weight:130.23
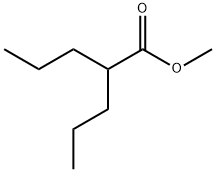
22632-59-3
42 suppliers
$175.00/1g

58175-57-8
29 suppliers
$32.50/1g
Yield:58175-57-8 81%
Reaction Conditions:
with Potassium borohydride;lithium chloride in tetrahydrofuran;acetic acid
Steps:
1.b (b)
(b) 2-n-Propyl-pentanol Into a perfectly dry flask, 800 to 850 ml of anhydrous tetrahydrofuran were introduced at room-temperature together with 81 g (1.5 mol) of potassium borohydride and 63.75 g (1.5 mol) of lithium chloride. Stirring was maintained for 30 minutes and then 158.2 g (1 mol) of crude methyl 2-n-propyl-pentanoate were introduced in one operation. The mixture was heated to reflux and so maintained for 4 hours. After cooling to room-temperature, 180 to 200 ml of glacial acetic acid were progressively introduced while maintaining the temperature ≤40° C. to obtain a pH=5-6. The medium was hydrolyzed with 1500 g of distilled water, the organic phase was decanted out and the aqueous phase was extracted with two fractions, each of 500 ml, of ethyl acetate. The organic phases were collected and washed with 2 fractions, each of 500 ml, of distilled water to neutrality. After drying on sodium sulphate, the solvent was eliminated with a rotatory evaporator. The crude alcohol so obtained was rectified at 100° C. under vacuum (p=50-55 mm Hg). In this manner 2-n-propyl-pentanol was obtained in a yield of 86%. Using the same method as that described above, 3-n-propyl-hexanol was prepared with a yield of 81%.
References:
Labaz US4246282, 1981, A
99-66-1
507 suppliers
$7.00/5g

58175-57-8
29 suppliers
$32.50/1g
17022-31-0
48 suppliers
inquiry

58175-57-8
29 suppliers
$32.50/1g
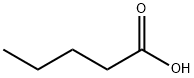
109-52-4
329 suppliers
$10.60/1gm:

58175-57-8
29 suppliers
$32.50/1g
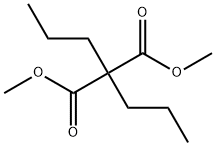
16644-05-6
21 suppliers
$45.00/10mg

58175-57-8
29 suppliers
$32.50/1g