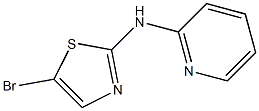
2-Pyridinamine, N-(5-bromo-2-thiazolyl)- synthesis
- Product Name:2-Pyridinamine, N-(5-bromo-2-thiazolyl)-
- CAS Number:54670-78-9
- Molecular formula:C8H6BrN3S
- Molecular Weight:256.1223
Yield:-
Reaction Conditions:
with water;sodium carbonate;tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in toluene at 100; for 12 h;
Steps:
10
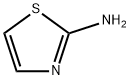
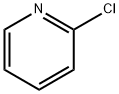
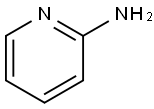
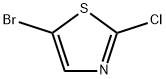
Example 10; (5-Bromo-thiazol-2-yiypyridin-2-yl-amme; A solution of 2-chloropyridine (0.34 g, 3.0 mmol), 2-aminothiazole (0.314 g,3.1 mmol), Na2CO3 (0.76 g, 7.2 mmol), Pd2(dba)3 (0.275 g, 0.3 mmol) and XantPhos (0.52 g, 0.9 mmol), H2O (54 mg, 3.0 mmol) in toluene (25 ml) is heated at 1000C for 12 hours. The reaction mixture is filtered and then concentrated in vacuum. The residue is purified by column chromatography (silica gel, eluted with CH2Cl2:Me0H:NH3 (7N, in MeOH) = EPO
References:
WO2007/16228,2007,A2 Location in patent:Page/Page column 23-24
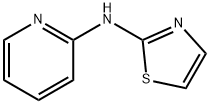
54670-80-3
7 suppliers
inquiry

54670-78-9
3 suppliers
inquiry
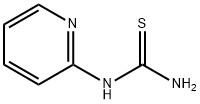
14294-11-2
133 suppliers
$8.00/1g

54670-78-9
3 suppliers
inquiry
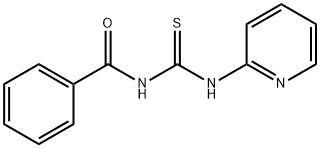
4921-86-2
57 suppliers
$28.00/1g

54670-78-9
3 suppliers
inquiry