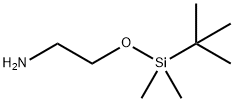
2-(tert-butyldiMethylsilyloxy)ethanaMine synthesis
- Product Name:2-(tert-butyldiMethylsilyloxy)ethanaMine
- CAS Number:101711-55-1
- Molecular formula:C8H21NOSi
- Molecular Weight:175.34

141-43-5
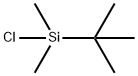
18162-48-6

101711-55-1
The general procedure for the synthesis of 2-((tert-butyldimethylsilyloxy)ethylamine from 2-aminoethanol and tert-butyldimethylsilyl chloride was as follows: a solution of tert-butyldimethylsilyl chloride (3.6 g, 24 mmol) dissolved in dichloromethane (10 mL) was added slowly and dropwise over a period of 3 minutes to a stirring mixture of 2-aminoethanol (1.22 g, 20 mmol) and imidazole (2.04 g, 30 mmol) in a mixed solution. The reaction mixture was continued to be stirred at room temperature for 1 hour. Subsequently, water (20 mL) was added for quenching and the organic and aqueous phases were separated. The aqueous phase was extracted with dichloromethane (2 x 20 mL), all organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give the light yellow oily product 2-((tert-butyldimethylsilyloxy)ethylamine (3.50 g, 100% yield). The product was confirmed by NMR hydrogen spectrum (400 MHz, CDCl3): δ 3.64 (t, J = 5.0 Hz, 2H), 3.05 (br s, 2H), 2.80 (t, J = 5.0 Hz, 2H), 0.90 (s, 9H), 0.06 (s, 6H); NMR carbon spectrum (100 MHz, CDCl3): δ 64.7, 44.1, 25.9, 18.3, -3.4; mass spectrum (APCI+) showing the molecular ion peak [M + H]+ = 176.6.

141-43-5
911 suppliers
$9.00/10g

18162-48-6
677 suppliers
$9.00/5g

101711-55-1
116 suppliers
$11.00/1g
Yield:101711-55-1 100%
Reaction Conditions:
with 1H-imidazole in dichloromethane at 20; for 1 h;
Steps:
1 2- ( (tert-butyldimethylsilyl) oxy) ethan-l-amine (4):
A solution of tert-butyldimethylchlorosilane (3.6 g, 24 mmol) and dichloromethane (10 mL) was added drop wise over 3 min to a stirred solution of ethanolamine (1.22 g, 20 mmol), imidazole (2.04 g, 30 mmol), and dichloromethane (20 mL) at room temperature, and the resulting mixture was stirred at room temperature for 1 h., water (20 mL) was added, and the phases were separated. The aqueous phase was extracted with dichloromethane (2 * 20 mL) , and the combined organic phases were dried (MgSOj) , filtered and concentrated in vacuo to give the title compound (3.50 g, 100%) as pale yellow oil. NMR (400 MH z , CDCI3 ) 6 3.64 (t, J = 5.0, 2H,), 3.05 (br s, 2H) , 2.80 (t, J = 5.0, 2H) , 0.90 (s, 9H), 0.06 (s, 6H) ; 13C NMR (100 MHz, CDCI3) δ 64.7, 44.1, 25.9, 18.3,-3.4; [M+H] * = 176.6 (APCI+) .
References:
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK;MEMORIAL SLOAN-KETTERING CANCER CENTER;BRESLOW, Ronald;MARKS, Paul;MAHENDRAN, Adaickapillai;YAO, Yuanshan WO2015/100363, 2015, A1 Location in patent:Page/Page column 43

18162-48-6
677 suppliers
$9.00/5g

101711-55-1
116 suppliers
$11.00/1g