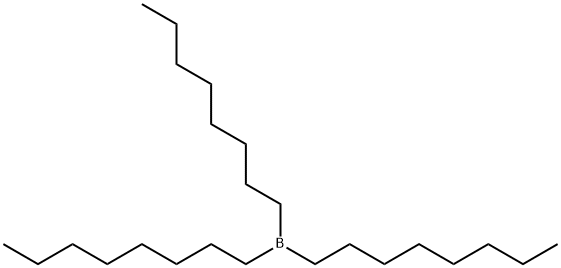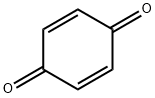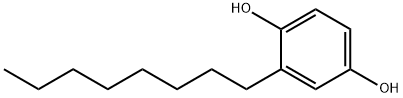
2-tert-Octylbenzene-1,4-diol synthesis
- Product Name:2-tert-Octylbenzene-1,4-diol
- CAS Number:1706-69-0
- Molecular formula:C14H22O2
- Molecular Weight:222.32
Yield:-
Reaction Conditions:
in diethyl ether;Inert atmosphere;
Steps:
4.1 2-Alkylhydroquinone synthesis method A (using BH3SMe2)
General procedure: The following procedure was adapted from a published procedure.13 A flame-dried three-neck 500-mL round-bottomed flask was charged with 0.050mol of borane dimethylsulfide (neat liquid) and 50mL ether. A nitrogen inlet, dropping funnel, magnetic stir bar, and condenser were fitted to the flask. Liquid alkene (0.15mol) was added to the dropping funnel and added dropwise to the stirred mixture, using 10mL of ether to rinse the funnel. The reaction can be slightly exothermic, depending on the alkene, and induce reflux. Once all of the alkene was added and reflux had subsided, the solvent was removed by distillation using a steam bath. 1,4-Benzoquinone (4.32g, 0.040mol) was then dissolved in 125mL of ether and added dropwise to the solution using the addition funnel. Upon complete addition of the 1,4-benzoquinone the nitrogen inlet was removed, and the ether was removed by distillation using a steam bath. Water (350mL) was added to the oil, and steam distillation was used to remove the dialkylborinic acid byproduct (R2BOH). Several additions of DI water were required for the steam distillation to completely remove the borinic acid byproduct, depending on the alkene, until the distillate showed no signal in an 11B-NMR spectroscopic analysis. The reaction flask was then cooled using an ice bath, causing the oily crude 2-alkylhydroquinone to precipitate as a chunky tan or brown solid. The solid was collected on a Buchner funnel, washed with water (100mL), and dried briefly under air. Analytically pure samples were obtained as white powders by recrystallization from toluene and hexanes (1:1).
References:
Kaurich, Kevin J.;Deck, Paul A. [Tetrahedron,2018,vol. 74,# 18,p. 2191 - 2196]
4693-19-0
1 suppliers
inquiry

1706-69-0
22 suppliers
inquiry
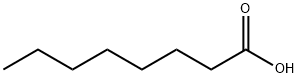
124-07-2
763 suppliers
$6.00/25g

1706-69-0
22 suppliers
inquiry