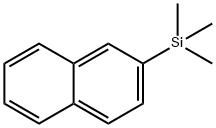
2-(TRIMETHYLSILYL)NAPHTHALENE synthesis
- Product Name:2-(TRIMETHYLSILYL)NAPHTHALENE
- CAS Number:18052-85-2
- Molecular formula:C13H16Si
- Molecular Weight:200.35
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

18052-85-2
17 suppliers
$412.00/1g
Yield: 76%
Reaction Conditions:
with potassium phosphate;chlorobis(ethylene)rhodium(I) dimer;C21H25N2(1+)*Cl(1-)*ClH;potassium tert-butylate;isopropyl alcohol in toluene at 180; for 16 h;Sealed tube;Inert atmosphere;Glovebox;
Steps:
IV. General Procedure for Rh-Catalyzed C-O Bond Reduction of Aryl Carbamates
General procedure: [RhCl(C2H4)2]2 (5.8 mg, 0.015 mmol), L2·HCl (21 mg, 0.060 mmol), KOtBu (7.4 mg, 0.066 mmol),K3PO4 (127 mg, 0.60 mmol), and toluene (0.40 mL) were added to a 5 mL screw-capped vial in aglovebox filled with nitrogen, and the resulting mixture was stirred at 60 °C for 1 h. Carbamate 1a(53 mg, 0.30 mmol), 2-propanol (18 mg, 0.30 mmol) and toluene (0.60 mL) were added to the vial inthe glove box. The vessel was stirred at 180 °C for 4 h followed by cooling to rt. The mixture waspurified by flash column chromatography over silica gel (eluting with hexane/EtOAc = 100/1) togive 2a as a white solid (36 mg, 78%).
References:
Yasui, Kosuke;Higashino, Masaya;Chatani, Naoto;Tobisu, Mamoru [Synlett,2017,vol. 28,# 19,p. 2569 - 2572] Location in patent:supporting information
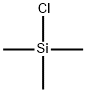
613-46-7
160 suppliers
$5.00/1g
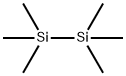
1450-14-2
287 suppliers
$29.12/10ML

18052-85-2
17 suppliers
$412.00/1g

1450-14-2
287 suppliers
$29.12/10ML
580-13-2
506 suppliers
$6.00/1g

18052-85-2
17 suppliers
$412.00/1g
![Naphthalene, 2-[(trimethylsilyl)carbonyl]-](/CAS/20210305/GIF/22364-54-1.gif)
22364-54-1
0 suppliers
inquiry

18052-85-2
17 suppliers
$412.00/1g