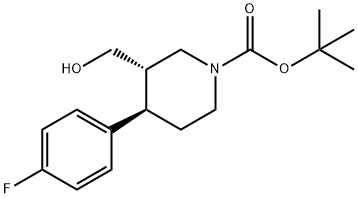
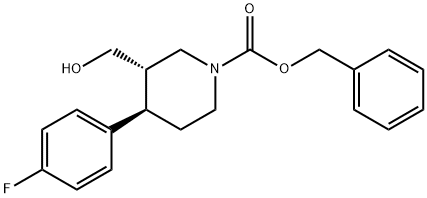
(3S,4R)-1-BOC-3-HYDROXYMETHYL-4-(4-FLUOROPHENYL)-PIPERIDINE synthesis
- Product Name:(3S,4R)-1-BOC-3-HYDROXYMETHYL-4-(4-FLUOROPHENYL)-PIPERIDINE
- CAS Number:200572-33-4
- Molecular formula:C17H24FNO3
- Molecular Weight:309.38
923932-21-2
11 suppliers
inquiry

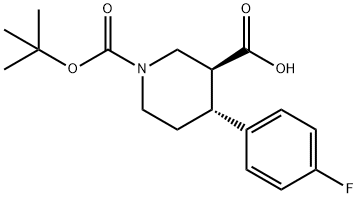
200572-33-4
39 suppliers
$226.00/250mg
Yield:200572-33-4 94%
Reaction Conditions:
Stage #1: (-)-(3S,4R)-4-(4-fluoro-phenyl)-piperidine-1,3-dicarboxylic acid 1-tert-butyl esterwith dimethylsulfide borane complex in tetrahydrofuran at 0 - 20;
Stage #2: with methanol in tetrahydrofuran at 0;
Steps:
1.d
d) (3S,4R)-4-(4-Fluoro-phenyl)-3-hydroxymethyl-piperidine-1-carboxylic acid tert-butyl ester To a solution of (-)-(3S,4R)-4-(4-fluoro-phenyl)-piperidine-1,3-dicarboxylic acid 1-tert-butyl ester (1.71 g, 5.29 mmol) in 35 ml THF was added a 2 M borane dimethylsulfide complex solution in THF (5.44 ml, 10.9 mmol) at 0° C. The mixture was stirred for 15 min at 0° C. and then at room temperature over night. After cooling to 0° C. the reaction was quenched by the addition of methanol. Stirring was continued until no evolution of gas was observed any more. The reaction mixture was diluted with water and extracted with 3 portions of tert-butyl methyl ethyl. The combined organic layers were dried over sodium sulfate and concentrated in vacuo. Flash chromatography gave 1.58 g (94%) of the title compound as a colorless viscous oil. MS m/e (%): 310 (M+H+, 32)
References:
US2007/232652,2007,A1 Location in patent:Page/Page column 15

24424-99-5
871 suppliers
$13.50/25G
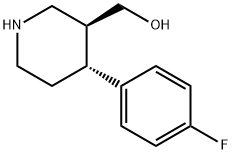
125224-43-3
99 suppliers
$49.00/25mg

200572-33-4
39 suppliers
$226.00/250mg

24424-99-5
871 suppliers
$13.50/25G
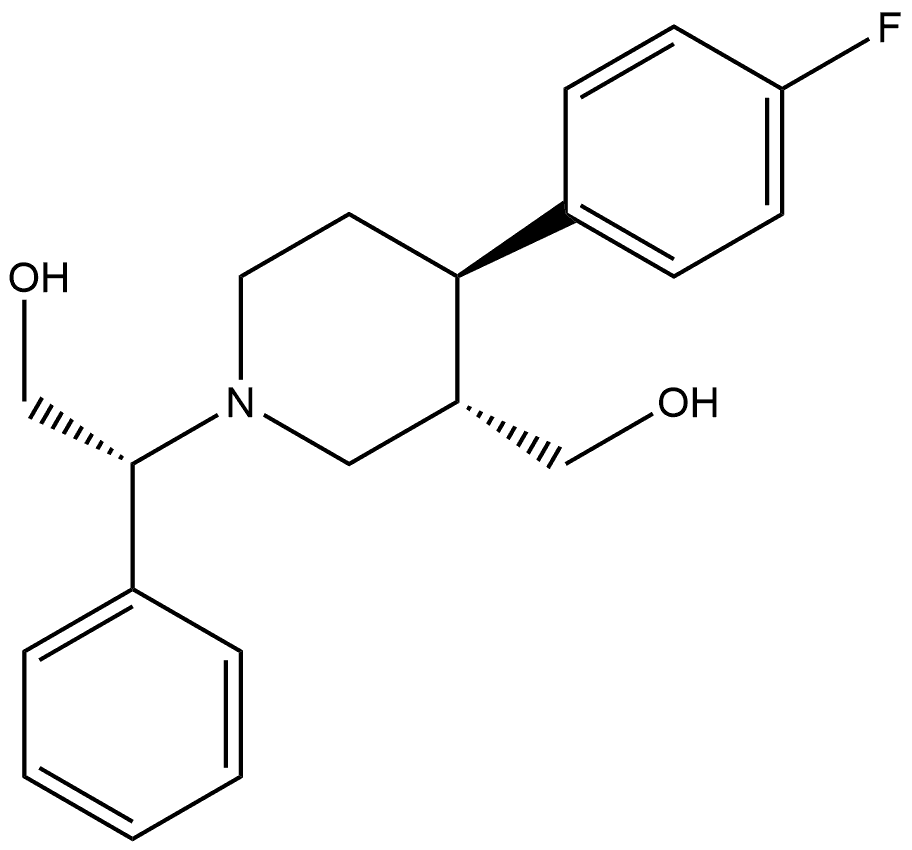
277744-08-8
0 suppliers
inquiry

200572-33-4
39 suppliers
$226.00/250mg

24424-99-5
871 suppliers
$13.50/25G
392328-26-6
4 suppliers
inquiry

200572-33-4
39 suppliers
$226.00/250mg
347-93-3
187 suppliers
$8.00/5g

200572-33-4
39 suppliers
$226.00/250mg