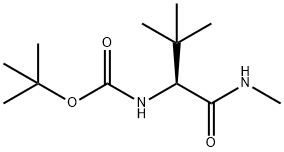
(S)-tert-butyl (3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl)carbamate(WXC08256) synthesis
- Product Name:(S)-tert-butyl (3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl)carbamate(WXC08256)
- CAS Number:200865-04-9
- Molecular formula:C12H24N2O3
- Molecular Weight:244.33
Yield:200865-04-9 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;HATU in DMF (N,N-dimethyl-formamide) at 0 - 20; for 4 h;
Steps:
12.A
The acid, 13001a, (5 g, 21.6 mmol, 1 equiv. Fluka) and methyl amine hydrochloride (1.2 equiv. , 25.92 mmol) were dissolved in dry N, N-dimethyl formamide (20 mi) and cooled to 0 °C. Added HATU (1.2 equiv. , 25.92 mmol) followed by DIPEA (Sigma-Aldrich), (172.8 mmol., 8 equiv. ) under N2 atmosphere. The temperature was slowly raised to room temperature and stirred further for 4 h at room temperature. Diluted with EtOAc and washed with 1 N HCI, NaHC03and finally with brine. Dried over anhydrous sodium sulfate, filtered, and evaporated off the solvent. Crude product isolated was purified via flash column (10-50% EtOAc-Hexane) to afford 5.27 g of 13001b. Yield, (99 %). 1H NMR (CDCI3, 300 MHz) 8, 6.0 (bs, 1 H), 5. 35 (d, 1 H, ), 3.82 (d, 1 H), 2.8 (s, 3H), 1.4 (s, 9H), 0.98 (s, 9 H).
References:
WO2005/87721,2005,A2 Location in patent:Page/Page column 224
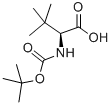
62965-35-9
445 suppliers
$6.00/5g
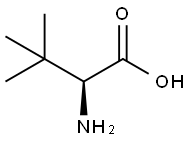
593-51-1
557 suppliers
$21.00/100g

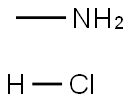
200865-04-9
16 suppliers
inquiry
20859-02-3
620 suppliers
$9.00/5G

200865-04-9
16 suppliers
inquiry

24424-99-5
863 suppliers
$13.50/25G

200865-04-9
16 suppliers
inquiry

20859-02-3
620 suppliers
$9.00/5G

24424-99-5
863 suppliers
$13.50/25G

200865-04-9
16 suppliers
inquiry
