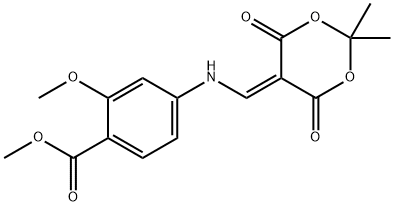
4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester synthesis
- Product Name:4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester
- CAS Number:205448-64-2
- Molecular formula:C16H17NO7
- Molecular Weight:335.31
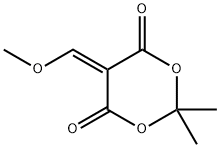
15568-85-1
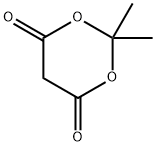
27492-84-8
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
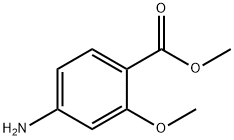
The general procedure for the synthesis of methyl 4-((2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-yl)methylamino)-2-methoxybenzoate from 5-(methoxymethylenyl)-2,2-dimethyl-1,3-dioxane-4,6-dione and 2-methoxy-4-aminobenzoic acid methyl ester was as follows: first, 2-methoxy-4-aminobenzoic acid methyl ester (14.15 g, 78 mmol) was suspended in isopropanol and heated at 50 °C. Subsequently, 5-(methoxymethylenyl)-2,2-dimethyl-1,3-dioxohexane-4,6-dione (14.8 g, 80 mmol) was added in batches over 10 min. The reaction mixture was heated to reflux for 30 minutes. After completion of the reaction, the mixture was cooled to room temperature and the precipitate formed was collected by filtration. The precipitate was washed with isopropanol and dried under vacuum to afford the target product 5-((3-methoxy-4-methoxycarbonylanilino)methylene)-2,2-dimethyl-1,3-dioxane-4,6-dione (25.2 g, 96% yield).
2033-24-1
664 suppliers
$6.00/25g

27492-84-8
314 suppliers
$6.00/5g
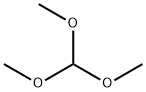
149-73-5
550 suppliers
$12.00/5g
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg
Yield:205448-64-2 89.62%
Reaction Conditions:
in isopropyl alcohol at 90; for 1 h;
Steps:
Methyl 2-methoxy-4-amino-benzoate (500.00 g, 2.76 mol), 2,2-dimethyl-1,3-dioxane-4,6-dione (397.73 g, 2.76 mol) and trimethyl orthoformate (292.84 g, 2.76 mol) were added to isopropanol (5.00 L). The reaction solution was heated up to an outer temperature of 90 °C and kept refluxing for 1 hour. The completion of the reaction was detected by TLC. The reaction solution was cooled to an outer temperature of 20 °C in an ice bath and then filtered. The solid was washed with MTBE (300 ml * 2) and then concentrated to dryness in a water bath. Compound 1A (873.00 g, 2.47 mol, the yield was 89.62% and the purity was 95%) was obtained as a light red powder. NMR (DMSO) demonstrated that the product was correct. 1H NMR (400 MHz, DMSO-d6) ppm 1.67 (s, 6 H) 3.76 (s, 3 H) 3.86 (s, 3 H) 7.19 (dd, J=8.41, 1.63 Hz, 1 H) 7.43 (d, J=1.25 Hz, 1 H) 7.71 (d, J=8.28 Hz, 1 H) 8.69 (d, J=10.04 Hz, 1 H) 11.25 (d, J=9.03 Hz, 1 H)
References:
GUANGDONG ZHONGSHENG PHARMACEUTICAL CO., LTD;LONG, Chaofeng;CHEN, Zhengxia;CHEN, Xiaoxin;ZHANG, Yang;LIU, Zhuowei;LI, Peng;CHEN, Shuhui;LIANG, Guibai;XIE, Cheng;LI, Zhengwei;FU, Zhifei;HU, Guoping;LI, Jian EP3293177, 2018, A1 Location in patent:Paragraph 0199; 0200

15568-85-1
161 suppliers
$7.00/1g

27492-84-8
314 suppliers
$6.00/5g
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg

2033-24-1
664 suppliers
$6.00/25g

27492-84-8
314 suppliers
$6.00/5g
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg

2033-24-1
664 suppliers
$6.00/25g

27492-84-8
314 suppliers
$6.00/5g
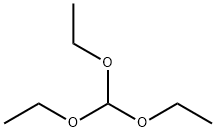
122-51-0
514 suppliers
$10.00/5ml
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg
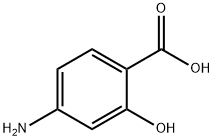
65-49-6
508 suppliers
$6.00/25g
![4-[(2,2-Dimethyl-4,6-dioxo-[1,3]dioxan-5-ylidenemethyl)-amino]-2-methoxy-benzoic acid methyl ester](/CAS/20180703/GIF/205448-64-2.gif)
205448-64-2
79 suppliers
$25.00/250mg