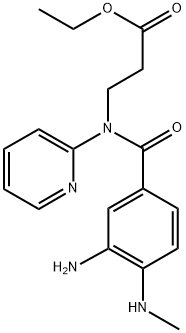
3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER synthesis
- Product Name:3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER
- CAS Number:212322-56-0
- Molecular formula:C18H22N4O3
- Molecular Weight:342.4
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
(D) Preparation of Intermediate VI: In a hydrogenation reactor, 43 g of ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzoylamino)propionate and 4.7 g of nickel Nguyenne catalyst were added. 4 mL of tetrahydrofuran was added as solvent and three nitrogen substitutions were performed to remove air. The reaction mixture was then stirred under hydrogen atmosphere and heated to 60°C. The reaction process was monitored by thin layer chromatography (TLC). After completion of the reaction, a filtration operation was carried out and the filter cake was washed with a small amount of tetrahydrofuran. The filter cake was recovered and disposed properly. The filtrate was dried over anhydrous sodium sulfate, diafiltrated again and finally concentrated to dryness under reduced pressure to give 38.3 g of intermediate VI (6) in 96% yield.
![ETHYL N-[4-(METHYLAMINO)-3-NITROBENZOYL]-N-PYRIDIN-2-YL-SS-ALANINATE](/CAS/GIF/429659-01-8.gif)
429659-01-8
195 suppliers
$12.00/5g
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
540 suppliers
$14.00/1 / G
Yield:212322-56-0 96%
Reaction Conditions:
with Raney nickel in tetrahydrofuran at 60;Inert atmosphere;
Steps:
3.d Example 3
(D) Preparation of intermediate VI: In a hydrogenation reaction flask,43 g of ethyl 3- (4- (methylamino) -3-nitro-N- (pyridin-2-yl) benzamido) propanoateAnd 4.7 g of Raney nickel in 500 mL of tetrahydrofuran,Nitrogen replacement 3 times, and then into the hydrogen, stirring, heating to 60 ° C reaction,TLC monitoring reaction; the reaction was completed, suction filtration, washing the filter cake with a small amount of tetrahydrofuran; the filter cake was recovered and applied; the filtrate was dried over anhydrous sodium sulfate, filtered with suction and concentrated to dryness under reduced pressure to give 38.3g Intermediate VI (6), yield 96%
References:
Nantong Changyou Pharmaceutical Technology Co., Ltd.;Li Zebiao;Yan Jun;Lin Yanfeng;Zou Lin;Zhao Yongxing CN104031031, 2017, B Location in patent:Paragraph 0028; 0033; 0035; 0040; 0042; 0047
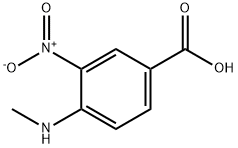
41263-74-5
321 suppliers
$9.00/5g
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
540 suppliers
$14.00/1 / G
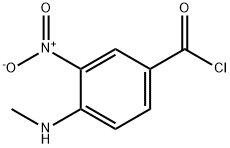
82357-48-0
37 suppliers
inquiry
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
540 suppliers
$14.00/1 / G
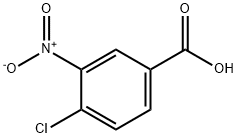
96-99-1
520 suppliers
$5.00/5g
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
540 suppliers
$14.00/1 / G
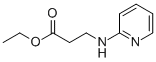
103041-38-9
374 suppliers
$6.00/1g
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](/CAS/GIF/212322-56-0.gif)
212322-56-0
540 suppliers
$14.00/1 / G