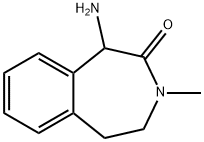
1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one synthesis
- Product Name:1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one
- CAS Number:253324-91-3
- Molecular formula:C11H14N2O
- Molecular Weight:190.24
![(Z)-1-(hydroxyiMino)-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-90-2.gif)
253324-90-2
11 suppliers
inquiry
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
17 suppliers
$65.00/5mg
Yield:253324-91-3 86%
Reaction Conditions:
with hydrogenchloride;hydrogen;5%-palladium/activated carbon in ethanol;water at 20; for 8.5 h;
Steps:
A4
[Example A4] Preparation of 1-amino-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one 42.0 g (168 mmol) of ethyl hydroxyimino-[2-(2-methylaminoethyl)phenyl]acetate obtained by the method of Example A2, 57 g (168 mmol) of sodium ethoxide-20% ethanol solution, and 126 ml of ethanol were added into a flask, and reacted at 50°C for five hours to obtain a solution of 1-(hydroxyimino)-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one. The reaction mixture was concentrated and then was added with 420 ml of 1N hydrochloric acid and 8.9 g of 5% palladium carbon. The mixture was treated under a hydrogen atmosphere at room temperature for 8.5 hours. The reaction mixture was filtered through a celite pad and the filtrate was extracted with dichloromethane under an alkaline condition. The extraction was concentrated to obtain 27.6 g (145 mmol, yield 86%) of 1-amino-3-methyl-1,3,4,5-tetrahydrobenz[d]azepin-2-one as a white solid. 1H-NMR (400 MHz, CDCl3) δ 3.02 (3H, s), 3.15 (1H, ddd, J=16.8, 10.3, 5.8 Hz), 3.25 (1H, dt, J=16.8, 5.1 Hz), 3.36 (1H, dt, J=14.6, 5.8 Hz), 4.14 (1H, ddd, J=14.6, 10.1, 4.6 Hz), 5.25 (1H, s), 7.09-7.13 (1H, m), 7.17-7.22 (1H, m), 7.23-7.28 (1H, m), 7.68-7.72 (1H, m).
References:
EP1985615,2008,A1 Location in patent:Page/Page column 18
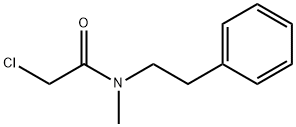
13230-84-7
2 suppliers
inquiry
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
17 suppliers
$65.00/5mg
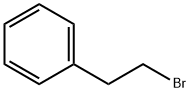
103-63-9
503 suppliers
$9.00/10g
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
17 suppliers
$65.00/5mg
![3-Methyl-1,3,4,5-tetrahydrobenzo[d]azepin-2-one](/CAS/GIF/73644-95-8.gif)
73644-95-8
27 suppliers
inquiry
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
17 suppliers
$65.00/5mg
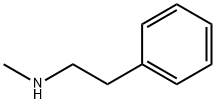
589-08-2
129 suppliers
$31.80/1gm:
![1-aMino-3-Methyl-4,5-dihydro-1H-benzo[d]azepin-2(3H)-one](/CAS/GIF/253324-91-3.gif)
253324-91-3
17 suppliers
$65.00/5mg