
1-Piperidinecarboxylic acid, 4-(1H-pyrazol-3-yl)-, 1,1-dimethylethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-(1H-pyrazol-3-yl)-, 1,1-dimethylethyl ester
- CAS Number:278798-07-5
- Molecular formula:C13H21N3O2
- Molecular Weight:251.32
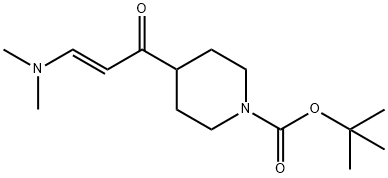
1799799-87-3
36 suppliers
inquiry

278798-07-5
22 suppliers
inquiry
Yield:278798-07-5 624 mg
Reaction Conditions:
with hydrazine hydrate in ethanol at 15 - 85;
Steps:
tert-butyl 4-(1H-pyrazol-3-yl)piperidine-1-carboxylate
0.23 ml. hydrazine monohydrate (4.8 mmol, CAS 7803-57-8) was added to a solution of 900 mg tert-butyl 4-[(2E)-3-(dimethylamino)prop-2-enoyl]piperidine-1 -carboxylate (3.19 mmol, intermediate 197) in 18 mL ethanol at 15°C. The reaction mixture stirred over night at 85°C and reaction progess was monitored by TCL (dichloromethane:ethanol 9:1 ; stained with potassium permanganate). The reaction mixture was evaporated and the crude product was purified by column chromatography (dichloromethane 100 -> dichloromethane:ethanol 90:10). The product was obtained in 78% yield (624 mg).1H NMR (400 MHz, DMSO-d6) d ppm 1 .40 (s, 9 H) 1 .44 (dd, 2 H) 1 .84 (br d, 2 H) 2.72 - 2.96 (m, 3 H) 3.96 (br d, 2 H) 6.05 (br d, 1 H) 7.22 - 7.68 (m, 1 H) 12.50 (br d, 1 H).
References:
WO2021/105117,2021,A1 Location in patent:Page/Page column 318

24424-99-5
859 suppliers
$13.50/25G

278798-07-5
22 suppliers
inquiry
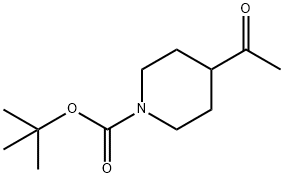
206989-61-9
219 suppliers
$10.00/1g

278798-07-5
22 suppliers
inquiry
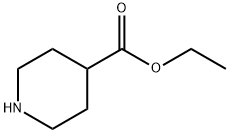
1126-09-6
478 suppliers
$15.40/25g

278798-07-5
22 suppliers
inquiry
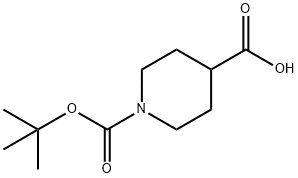
84358-13-4
378 suppliers
$5.00/5g

278798-07-5
22 suppliers
inquiry