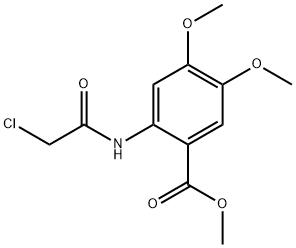
METHYL 2-[(2-CHLOROACETYL)AMINO]-4,5-DIMETHOXYBENZOATE synthesis
- Product Name:METHYL 2-[(2-CHLOROACETYL)AMINO]-4,5-DIMETHOXYBENZOATE
- CAS Number:285138-76-3
- Molecular formula:C12H14ClNO5
- Molecular Weight:287.7
26759-46-6
209 suppliers
$6.00/5g

79-04-9
382 suppliers
$12.00/5g
![METHYL 2-[(2-CHLOROACETYL)AMINO]-4,5-DIMETHOXYBENZOATE](/CAS/GIF/285138-76-3.gif)
285138-76-3
14 suppliers
$195.30/100MG
Yield:285138-76-3 98.7%
Reaction Conditions:
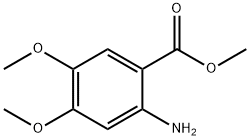
Stage #1: methyl 2-amino-4,5-dimethoxybenzoatewith potassium carbonate in acetone at 20; for 0.5 h;
Stage #2: chloroacetyl chloride in acetone at 0 - 20; for 3 h;
Steps:
5.1.1 General procedures for Scheme 1
General procedure: Step 1. To the stirred solution of Aniline (1.0 equiv) in acetone was added K2CO3 (1.2 equiv.) at RT and stirred for 30min. Chloroacetyl chloride/substituted acetylchloride (1.1 equiv.) was then added at 0°C and stirred for additional 3h at room temperature. After completion of the reaction (monitored by TLC), volatiles were removed on rotary evaporator and ethyl acetate was added. Organic layer were washed with water, aq NaHCO3, brine. Organic layer was dried over sodium sulfate and evaporated on rotary evaporator and the crude residue was purified by flash chromatography to give the desired product.
References:
Gangar, Mukesh;Goyal, Sandeep;Raykar, Digambar;Khurana, Princy;Martis, Ashwita M.;Goswami, Avijit;Ghoshal, Ishani;Patel, Ketul V.;Nagare, Yadav;Raikar, Santosh;Mukherjee, Apurba;Cyriac, Rajath;Paquin, Jean-Fran?ois;Kulkarni, Aditya [Bioorganic Chemistry,2022,vol. 119,art. no. 105549] Location in patent:supporting information