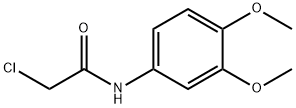
2-CHLORO-N-(3,4-DIMETHOXY-PHENYL)-ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(3,4-DIMETHOXY-PHENYL)-ACETAMIDE
- CAS Number:62593-78-6
- Molecular formula:C10H12ClNO3
- Molecular Weight:229.66

79-04-9
382 suppliers
$12.00/5g
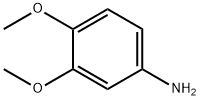
6315-89-5
314 suppliers
$7.00/10g

62593-78-6
26 suppliers
$45.00/25mg
Yield:62593-78-6 95%
Reaction Conditions:
with pyridine in dichloromethane at 20; for 12 h;
Steps:
1.1
General procedure for the synthesis of 2-halo-N-arylacetamides (2a-2i)
General procedure: The solution of arylamine (1.0 equiv.) and pyridine (1.0 equiv.) in DCM (concentration, 0.4 M) was cooled in an ice bath. Thesolution of 2-chloroacetyl chloride or 2-bromoacetyl bromide (1.1 equiv.) in DCM (concentration, 0.5 M) was added dropwise to theabove solution according to the references [1-3]. The resulting reaction mixture was warmed to room temperature and stirred for 12 h.The reaction mixture was diluted with DCM and then washed with 1M HCl and brine. The organic layer was dried over anhydrousMgSO4, filtered and concentrated in vacuo. The crude product was purified by crystallization from a mixture of solvent DCM andpetroleum ether.
References:
Zhao, Hai-Long;Zhou, Jie;Song, Hong-Rui;Xu, Bai-Ling [Chinese Chemical Letters,2014,vol. 25,# 3,p. 411 - 414] Location in patent:supporting information