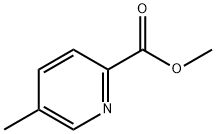
2-Pyridinecarboxylicacid,5-methyl-,methylester(9CI) synthesis
- Product Name:2-Pyridinecarboxylicacid,5-methyl-,methylester(9CI)
- CAS Number:29681-38-7
- Molecular formula:C8H9NO2
- Molecular Weight:151.16

67-56-1
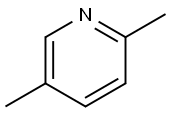
589-93-5

29681-38-7
Steps for the synthesis of Intermediate 20-6: Methyl 5-methylpyridine-2-carboxylate: Selenium dioxide (4.66 g, 42.0 mmol, CAS 7446-08-4) was added to a solution of 2,5-dimethylpyridine (3.00 g, 28.0 mmol, CAS 589-93-5) dissolved in pyridine (15 mL). The reaction mixture was heated to reflux overnight. After completion of the reaction, it was cooled to room temperature, the solid precipitate was removed by filtration and washed sequentially with water and pyridine (5 mL each, 2 times). The filtrate was evaporated and the crude product obtained was redissolved in methanol (100 mL). Sulfuric acid (1.34 mL, 25.0 mmol) was added to this solution and the reaction mixture was then heated to reflux for 5 hours. At the end of the reaction, it was cooled to room temperature and the reaction mixture was alkalized with 20% NaOH aqueous solution. Methanol was removed by evaporation and diluted by adding water (50 mL). The mixture was extracted with diethyl ether (DEE, 3 x 100 mL). The organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4), and the solvent was evaporated to give the title compound methyl 5-methylpyridine-2-carboxylate (2.11 g, 50% yield) as a light brown oil.1H NMR (400 MHz, CDCl3) δ 2.40 (s, 3H), 3.97 (s, 3H), 7.61 (d, 1H), 8.01 (d , 1H), 8.54 (s, 1H).

67-56-1
790 suppliers
$7.29/5ml-f

589-93-5
140 suppliers
$32.00/5mL

29681-38-7
114 suppliers
$13.00/100mg
Yield: 50%
Reaction Conditions:
Stage #1:2,5-dimethylpyridine with selenium(IV) oxide in pyridineReflux;
Stage #2:methanol with sulfuric acid for 5 h;Reflux;
Steps:
20.6
Intermediate 20-6: Methyl 5-methylpyridine-2-carboxylateTo a solution of 2,5-dimethylpyridine (3.00 g, 28.0 mmol, CAS 589-93-5) in pyridine (15 mL) was added selenium dioxide (4.66 g, 42.0 mmol, CAS 7446-08-4). The reaction mixture was heated at reflux overnight. After cooling to RT a solid was filtered off and washed with water and pyridine (2x5 mL/wash). The filtrate was evaporated and the crude retaken in methanol (100 mL). Sulphuric acid (1.34 mL, 25.0 mmol) was added and the reaction mixture was heated at reflux for 5 h. After cooling to RT the reaction mixture was basified with 20% NaOH (aq). The methanol was evaporated off and water was added (50 mL). This mixture was extracted with DEE (3 x 100 mL). The combined organics were dried (Na2SO4) and evaporated to afford the title compound (2.11 g, 50 %) as light-brown oil.1H NMR (400 MHz, CDCl3) δ 2.40 (s, 3H), 3.97 (s, 3H), 7.61 (d, IH), 8.01 (d, IH), 8.54 (s, IH).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2009/81195, 2009, A1 Location in patent:Page/Page column 84

67-56-1
790 suppliers
$7.29/5ml-f
4434-13-3
143 suppliers
$21.00/250mg

29681-38-7
114 suppliers
$13.00/100mg
3510-66-5
481 suppliers
$5.00/5g
201230-82-2
1 suppliers
inquiry

29681-38-7
114 suppliers
$13.00/100mg
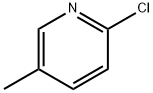
18368-64-4
455 suppliers
$10.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

29681-38-7
114 suppliers
$13.00/100mg

124-38-9
134 suppliers
$175.00/23402
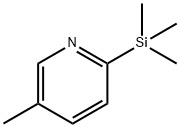
13737-09-2
12 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

29681-38-7
114 suppliers
$13.00/100mg