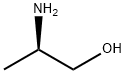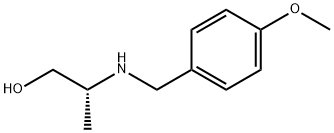
(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol synthesis
- Product Name:(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol
- CAS Number:570398-17-3
- Molecular formula:C11H17NO2
- Molecular Weight:195.26
Yield: 100%
Reaction Conditions:
Stage #1:(R)-2-aminopropan-1-ol;4-methoxy-benzaldehyde in methanol for 0.5 h;
Stage #2: with sodium tetrahydroborate in methanol at 0 - 20; for 1 h;
Stage #3: with water in methanolProduct distribution / selectivity;
Steps:
8; 18
Example 8; 3-Methyl-2-((3R)-3-methylmorpholin-4-yl)-6-(pyrimidin-4-yl)-3H- pyrimidin-4-one (Compound No. B63); (2R) - 2 - (4-Methoxvb enzylamino) -p r op an- 1 - ol; 4-Anisaldehyde (18.1 g, 133 mmol) was added to a solution of D-alaninol(lO g, 133 mmol) in methanol (100 ml) with vigorous stirring and the mixture was stirred for 30 minutes. Sodium borohydride (5.0 g, 132 mmol) was added to the ice-cooled solution and stirred for one hour at room temperature. When the reaction was complete (checked by thin layer chromatography), excess reagent was decomposed by the addition of water. The solution was partitioned between water and chloroform. The organic layer was washed with brine, dried over magnesium sulfate. Removal of the solvent under reduced pressure afforded (2R)-2-(4-Methoxybenzylamino)-propan- l-ol (26 g, 133 mmol, 100%) as white crystals.; Example 18; 6-(3-Fluoropyridin-4-yl)-3-methyl-2-((2R,5R)-cis-5-methyl-2- phenylmorpholin-4-yl);3H-pyrimidin-4-one; (2R)-2-(4-Methoxvbenzvlamino)-propan-l-ol; 4-Anisaldehyde (5.5 g, 40.4 mmol) was added to a solution of D-alaninol (3.0 g, 39.9 mmol) in methanol (30 ml) with, vigorous stirring and the mixture was stirred for 30 minutes. Sodium borohydride (1.52 g, 40.2 mmol) was added to the ice-cooled solution and stirred for 1 hour at room temperature. When the reaction was complete (checked by thin layer chromatography), excess reagent was decomposed by the addition of water. The solution was partitioned between water and chloroform. The organic layer was washed with brine, dried over magnesium sulfate. Removal of the solvent under reduced pressure afforded (2R)-2-(4-methoxybenzylamino)-propan-l-ol (7.79 g, 39.9 mmol, 100%) as white crystals.
References:
MITSUBISHI PHARMA CORPORATION;SANOFI-AVENTIS WO2007/119463, 2007, A1 Location in patent:Page/Page column 197; 215-216
![1-Propanol,2-[[(4-methoxyphenyl)methylene]amino]-,(2R)-(9CI)](/CAS/GIF/697763-78-3.gif)
697763-78-3
2 suppliers
inquiry
![(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol](/CAS/20180808/GIF/570398-17-3.gif)
570398-17-3
4 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol](/CAS/20180808/GIF/570398-17-3.gif)
570398-17-3
4 suppliers
inquiry
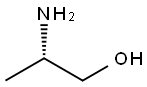
2749-11-3
416 suppliers
$14.56/5G
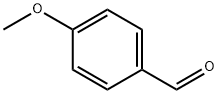
123-11-5
883 suppliers
$10.00/10g
![(2S)-2-[[(4-Methoxyphenyl)methyl]amino]-1-propanol](/CAS/20210111/GIF/570398-18-4.gif)
570398-18-4
2 suppliers
inquiry
![(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol](/CAS/20180808/GIF/570398-17-3.gif)
570398-17-3
4 suppliers
inquiry

123-11-5
883 suppliers
$10.00/10g
![(2R)-2-{[(4-methoxyphenyl)methyl]amino}propan-1-ol](/CAS/20180808/GIF/570398-17-3.gif)
570398-17-3
4 suppliers
inquiry