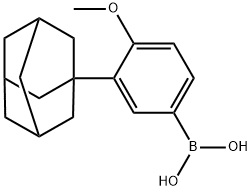
3-(1-ADAMANTYL)-4-METHOXYBENZENEBORONIC ACID synthesis
- Product Name:3-(1-ADAMANTYL)-4-METHOXYBENZENEBORONIC ACID
- CAS Number:459423-32-6
- Molecular formula:C17H23BO3
- Molecular Weight:286.17
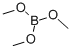
121-43-7
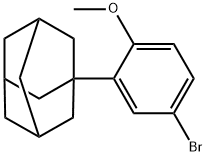
104224-63-7

459423-32-6
Step B. Synthesis of 3-(1-adamantyl)-4-methoxyphenylboronic acid Example 2: In a 1L three-necked flask equipped with a stirrer, reflux condenser, and dropping funnel, a mixture of 8 g (0.33 mol) of magnesium shavings and 200 mL of anhydrous tetrahydrofuran was added. Subsequently 8 g (0.19 mol) of crushed anhydrous lithium chloride was added. The air in the flask was replaced with argon and subsequent operations were carried out under an inert atmosphere. Under vigorous stirring, 11 g (5 mL, 0.06 mol) of 1,2-dibromoethane was added all at once. After the vigorous reaction subsided, 50 g (0.16 mol) of 2-(1-adamantyl)-4-bromoanisole dissolved in 400 mL of tetrahydrofuran was slowly added dropwise, with the rate of titration controlled to maintain a slight reflux. After dropwise addition, stirring was continued and heated to slow reflux for 30 minutes. Stirring was stopped and the resulting Grignard reagent was decanted from the residual magnesium into a conical flask that had been pre-flushed with argon and cooled to 0°C and kept sealed for 2 hours. In another 1L three-necked flask, 33 g (36 mL, 0.35 mol) of trimethyl borate and 100 mL of anhydrous tetrahydrofuran were added and mixed with stirring. The resulting solution was cooled to 0-5 °C (ice-water bath) and the pre-prepared Grignard reagent was added dropwise over 10 min under vigorous stirring. The reaction mixture was allowed to stand overnight (16 hours) in a refrigerator at 0-5°C. Subsequently, cooling was withdrawn and 50 mL of hydrochloric acid (diluted in 50 mL of water) was added under vigorous stirring to quench the reaction. The mixture was transferred to a partitioning funnel for layering. The aqueous layer was diluted with 200 mL of water and extracted twice with 100 mL of diethyl ether. The organic phases were combined, dried with anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent (water bath temperature about 50°C). The residue was treated with 200 mL of ethyl acetate and allowed to stand overnight in a refrigerator. The precipitate was collected by filtration and dried at 100 °C to give 30 g of 3-(1-adamantyl)-4-methoxyphenylboronic acid (68% yield) with a melting point of 300 °C. Example 10: The reaction was carried out according to the method described in Example 2, with the difference that the reaction of 3-(1-adamantyl)-4-methoxyphenylmagnesium bromide solution with trimethyl borate was carried out at -70°C, to ultimately obtain 30.6 g of 3-(1-adamantyl)-4-methoxyphenylboronic acid (69% yield).
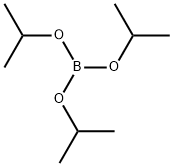
5419-55-6
387 suppliers
$12.00/5g

459423-32-6
99 suppliers
$50.00/25mg
Yield: 94.8%
Reaction Conditions:
Stage #1:2-(1-adamantyl)-4-bromoanisole with n-butyllithium in tetrahydrofuran at -75 - -70; for 1 h;
Stage #2:Triisopropyl borate in tetrahydrofuran at -70 - 20;
Stage #3: with hydrogenchloride;water in tetrahydrofuran at 20;
Steps:
1.a
Example 1 : EPO
References:
GALDERMA RESEARCH & DEVELOPMENT, S.N.C. WO2006/108717, 2006, A2 Location in patent:Page/Page column 8-9

104224-63-7
303 suppliers
$16.00/5g

459423-32-6
99 suppliers
$50.00/25mg
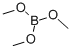
121-43-7
381 suppliers
$13.00/25mL

104224-63-7
303 suppliers
$16.00/5g

459423-32-6
99 suppliers
$50.00/25mg
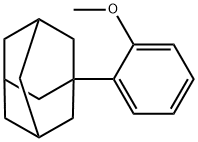
43109-77-9
75 suppliers
$100.00/50mg
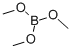
121-43-7
381 suppliers
$13.00/25mL

104224-63-7
303 suppliers
$16.00/5g

459423-32-6
99 suppliers
$50.00/25mg