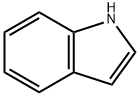
3-(2-Chloro-5-methylpyrimidin-4-yl)-1H-indole synthesis
- Product Name:3-(2-Chloro-5-methylpyrimidin-4-yl)-1H-indole
- CAS Number:945016-62-6
- Molecular formula:C13H10ClN3
- Molecular Weight:243.69
Yield:945016-62-6 54.2%
Reaction Conditions:
with methyl magnesium iodide in diethyl ether;1,2-dichloro-ethane at 0;Inert atmosphere;
Steps:
Intermediate 1 3-(2-Chloro-5-methylpyrimidin-4-yl)-1H-indole Methylmagnesium iodide (3 M in diethyl ether, 30.7 mL, 92.02 mmol) was added over 20 min to a solution of 1H-indole (7.19 g, 61.35 mmol) in DCE (25 mL) under nitrogen at 0° C. 2,4-Dichloro-5-methylpyrimidine (10 g, 61.35 mmol) in DCE (20 mL) was then added slowly to the reaction mixture over 20 min at 0° C. and the reaction mixture was stirred for an additional 30 min at 0° C. The reaction mixture was then allowed to warm up to RT and stirred at 30 min. The reaction mixture was then cooled to 0° C. and water (50 mL) was slowly added. The resultant yellow solids were collected by vacuum filtration. The solids were then suspended in 10% aqueous citric acid solution (150 mL) and stirred for 10 min, filtered, then washed with water and diethyl ether to give the title product. (8.10 g, 54.2%) as a yellow solid.1H NMR (300 MHz, CDCl3) δ ppm 10.74 (br. s., 1H), 8.47-8.44 (m, 1H), 8.22 (s, 1H), 7.73 (d, 1H), 7.40-7.36 (m, 1H), 7.21-7.17 (m, 2H), 2.39 (s, 3H). m/z 244.
References:
US2012/28924,2012,A1 Location in patent:Page/Page column 24
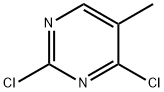
1780-31-0
362 suppliers
$10.00/5g

945016-62-6
8 suppliers
inquiry
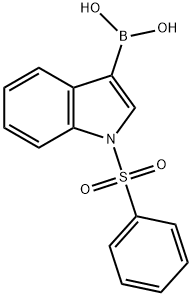
129271-98-3
98 suppliers
$15.00/100mg

945016-62-6
8 suppliers
inquiry