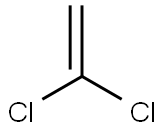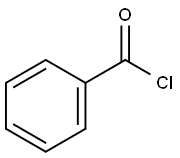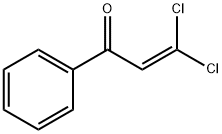
3,3-DICHLORO-1-PHENYL-2-PROPEN-1-ONE, 97 % synthesis
- Product Name:3,3-DICHLORO-1-PHENYL-2-PROPEN-1-ONE, 97 %
- CAS Number:10562-42-2
- Molecular formula:C9H6Cl2O
- Molecular Weight:201.05
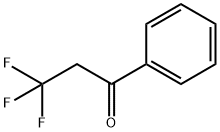
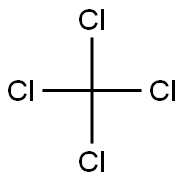
709-21-7
39 suppliers
$45.00/250mg

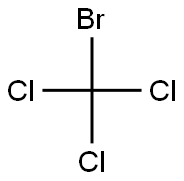
10562-42-2
3 suppliers
inquiry
Yield:10562-42-2 79%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 0 - 20; for 120 h;Sealed tube;Inert atmosphere;
Steps:
General procedure B for the preparation of 1,1-dichloro-1-alkenes
General procedure: The corresponding 1,1,1-trifluoroalkanone (200 mg, 1.00 equiv) was dissolved in CH2Cl2 (3 mL) in aRadley tube under argon. To this solution was added AlCl3 (542 mg, 5.00 equiv) at 0 °C. The solutionwas stirred at room temperature for 0.5-2.5 h unless stated otherwise. Upon completion of thereaction (monitored by LCMS) the reaction was quenched with 2M HCl and extracted with CH2Cl2 (3 ×10 mL). The combined organic layers were dried (MgSO4), filtered, and concentrated. If required, theresulting residue was purified by either flash column chromatography (15 g silica, 0-100%heptane/ethyl acetate) or reversed-phase column chromatography (12 g silica, 0-100%water/acetonitrile).
References:
Lansbergen, Beatrice;Meister, Catherine S.;McLeod, Michael C. [Beilstein Journal of Organic Chemistry,2021,vol. 17,p. 404 - 409] Location in patent:supporting information
![Silane, (1,1-dimethylethyl)dimethyl[(1-phenylethenyl)oxy]-](/CAS/20180529/GIF/66324-10-5.gif)