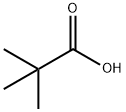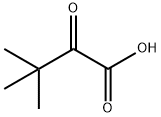
3,3-Dimethyl-2-oxobutyric acid synthesis
- Product Name:3,3-Dimethyl-2-oxobutyric acid
- CAS Number:815-17-8
- Molecular formula:C6H10O3
- Molecular Weight:130.14
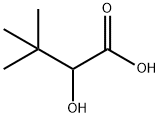
4026-20-4

815-17-8
The general procedure for the synthesis of trimethylpyruvic acid (DOBA) from 2-hydroxy-3,3-dimethylbutyric acid (DHBA) is as follows: 1. dissolve 0.1 mol (13.2 g) of 3,3-dimethyl-2-hydroxybutyric acid (DHBA) in 100 mL of 5 N sodium hydroxide solution (containing 5 mol NaOH/mol DHBA). 2. 5 x 10^-4 mol Bi(NO3)3/mol DHBA was added as an additive and the reaction mixture was heated to about 100 °C at atmospheric pressure in the presence of 1 g of activated carbon containing 5 wt. % palladium (pharmaceutical charcoal) until about 1,300 mL of oxygen (about 0.535 mol O2/mol DHBA) was absorbed. 3. The reaction times and results of 3,3-dimethyl-2-oxobutyric acid (DOBA) yields determined by polarography are summarized in Table 2. Following the procedure of Example 1, 0.1 mol (13.2 g) of 3,3-dimethyl-2-hydroxybutyric acid (DHBA) was dissolved in 100 mL of sodium hydroxide solution at various concentrations, 5 x 10^-4 mol Bi(NO3)3/mol DHBA was added as an activator, and the reaction was carried out in the presence of 1 g of activated charcoal containing 5 wt. % palladium at 95 °C and atmospheric pressure until about 1,300 mL of oxygen (about 0.535 mol O2/mol DHBA) was absorbed. The reaction time and DOBA yield results are summarized in Table 3. Example 16 is a control experiment in which no super stoichiometric base was used.
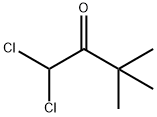
22591-21-5
187 suppliers
$5.00/5g

815-17-8
287 suppliers
$41.00/25g
Yield:815-17-8 200 kg
Reaction Conditions:
Stage #1:1,1-dichloro-3,3-dimethylbutan-2-one with sodium hydroxide in water for 3 h;Large scale;
Stage #2: with potassium permanganate in water at 55; for 8 h;Large scale;Temperature;
Steps:
2 Example 2
1) Hydrolysis reaction: To a 2000 L enamel reactor, 300 kg of dichloro-pyridone and 300 kg of water were added,Stirring warming,Heating to 70 after the insulation,5h dropping 1000kg concentration of 30% of the liquid base,Liquid base added to continue after the reaction 3h,Obtaining an aqueous solution containing the intermediate for use;2) Oxidation reaction: 250 kg of potassium permanganate was added to the aqueous intermediate solution obtained in step 1) in five portions,Each batch plus 50kg, during the temperature control 55 ,5h, after adding potassium permanganate, continue to react after 3h to end the reaction,Filtration, collecting filtrate,The filtrate was adjusted to pH = 1 with concentrated hydrochloric acid,After adjusting the pH,The filtrate was extracted with 500 kg of dichloromethane to obtain an organic layer,The organic layer was distilled at atmospheric pressure to 100 ° C,And then cooled to 40 ,Slowly add 300kg petroleum ether temperature 8 stirring reaction 5h,Centrifugal reaction liquid,The product 200kg.
References:
Huzhou Hengyuan Biochem. Tech. Co., Ltd.;Ma, Weiheng CN105884606, 2016, A Location in patent:Paragraph 0024; 0025; 0026; 0027

4026-20-4
24 suppliers
inquiry

815-17-8
287 suppliers
$41.00/25g
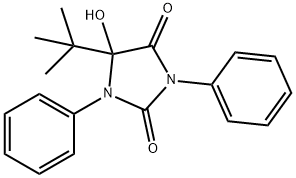
1352131-48-6
0 suppliers
inquiry

815-17-8
287 suppliers
$41.00/25g
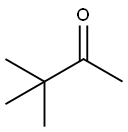
75-97-8
245 suppliers
$10.00/1g

815-17-8
287 suppliers
$41.00/25g