3-(4-FLUOROPHENYL)CYCLOPENTANONE synthesis
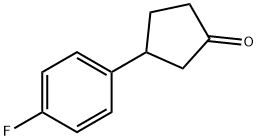
- Product Name:3-(4-FLUOROPHENYL)CYCLOPENTANONE
- CAS Number:165591-10-6
- Molecular formula:C11H11FO
- Molecular Weight:178.2
Yield:165591-10-6 96%
Reaction Conditions:
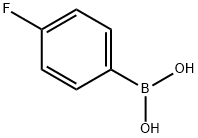
Stage #1: 4-fluoroboronic acidwith chloro(1,5-cyclooctadiene)rhodium(I) dimer;sodium carbonate in water; for 0.005 h;Inert atmosphere;
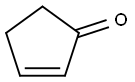
Stage #2: cyclopent-2-enone in water at 80; for 1 h;
Steps:
1.1 Step 1:
Step 1:
3-(4-Fluorophenyl)cyclopentan-1-one
A vial charged with [Rh(cod)C1]2 (180.2 mg, 0.3654 mmol) and (4-fluorophenyl)boronic acid (4.261 g, 30.45 mmol) was flushed with nitrogen.
To the reaction vial was added sequentially, water (60 mL) (degassed for >1 hr with nitrogen) followed by sodium carbonate (2.582 g, 24.36 mmol).
The mixture was stirred under a nitrogen atmosphere until the sodium carbonate was fully dissolved (?3 min). Cyclopent-2-en-1-one (1.0 g, 12.18 mmol) was added to the mixture.
The heterogeneous mixture was heated to 80° C. under a nitrogen atmosphere.
After 1 hr, the reaction was cooled to room temperature.
The aqueous mixture was extracted with ethyl acetate (2*) and again with dichloromethane (2*).
The combined organic layers were dried over anhydrous sodium sulfate, filtered through a short plug of silica gel (-8 gram) and concentrated to provide the desired product wt. 2.09 g, 96%.yield. 1H NMR (400 MHz, CDCl3) δ 7.23-7.14 (m, 1H), 7.01 (dd, J=12.0, 5.3 Hz, 1H), 3.39 (ddd, J=18.1, 11.1, 6.9 Hz, 1H), 2.66 (dd, J=18.0, 7.6 Hz, 1H), 2.54-2.36 (m, 1H), 2.29 (dt, J=11.1, 9.9 Hz, 1H), 2.03-1.84 (m, 1H).
References:
US2019/322673,2019,A1 Location in patent:Paragraph 0478; 0723-0724
143589-42-8
18 suppliers
inquiry

165591-10-6
14 suppliers
inquiry

143589-79-1
0 suppliers
inquiry

165591-10-6
14 suppliers
inquiry