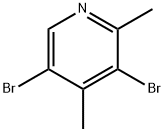
3,5 - DibroMo - 2,4 - diMethylpyridine synthesis
- Product Name:3,5 - DibroMo - 2,4 - diMethylpyridine
- CAS Number:29976-20-3
- Molecular formula:C7H7Br2N
- Molecular Weight:264.95
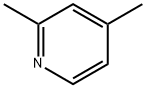
108-47-4
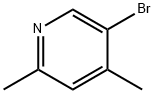
27063-92-9

29976-20-3
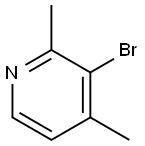
27063-93-0
GENERAL STEPS: Dissolve 2,4-dimethylpyridine (5.39 mL, 46.7 mmol, 1 eq.) in bromide (50 mL) containing 20% free SO3 at 0 °C with stirring. A high efficiency reflux condenser was installed and heated to 165°C in an oil bath. Bromine (4.30 mL, 83.4 mmol, 0.9 eq.) was added in batches over a period of 5 hours, maintaining the temperature at 155-175 °C and stirring for 20 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was poured into crushed ice (200 g) and stirred for 1 hour. The solution was neutralized with solid Na2CO3 vesicles to an orange solution, diluted with water and extracted with EtOAc (2 x 250 mL). The organic layers were combined, dried with Na2SO4 and concentrated in vacuum. The crude product was purified by automated fast column chromatography (Biotage KP-Sil SNAP 340g column, 0-15% EtOAc/hexane) and dried under vacuum to give three brominated products: 3,5-dibromo-2,4-dimethylpyridine (8), 5-bromo-2,4-dimethylpyridine (9) and 3-bromo-2,4-dimethylpyridine (10). 3,5-Dibromo-2,4-dimethylpyridine (8) is a white solid (1.48 g, 12%) with a melting point of 28-30 °C. 1H NMR (400 MHz, CDCl3) δ 2.54 (s, 3H, CH3), 2.61 (s, 3H, CH3), 8.41 (s, 1H, pyHortho); 13C NMR (100 MHz, CDCl3) δ 23.8 (CH3), 25.7 (CH3), 120.3 (ArC), 124.4 (ArC), 146.4 (ArC), 148.5 (ArCH), 156.5 (ArC); IR νmax (film)/cm?1 3045, 2998, 2956, 2921, 1558, 1433, 1376, 1349, 1232, 1376, 1349, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232, 1232 1376, 1349, 1232, 1049, 993, 928, 783; HRMS (ESI+) m/z calcd for C7H8Br2N [M+H]+ 263.9018, found 263.9027. 5-Bromo-2,4-dimethylpyridine (9) was a colorless oil (1.89 g, 22%).1H NMR (400 MHz, CDCl3) δ 2.32 (s, 3H, CH3), 2.44 (s, 3H, CH3), 7.00 (s, 1H, pyHortho), 8.47 (s, 1H, pyHmeta); 13C NMR ( 100 MHz, CDCl3) δ 22.1 (CH3), 23.6 (CH3), 120.4 (ArC), 125.5 (ArCH), 146.7 (ArC), 150.5 (ArCH), 157.0 (ArC); IR νmax (film)/cm?1 2985, 2957, 2924, 2854, 1592, 1461, 1377 1592, 1461, 1377, 1353, 1289, 1177, 1032; HRMS (ESI+) m/z calcd for C7H9BrN [M+H]+ 185.9913, found 185.9918. 3-Bromo-2,4-dimethylpyridine (10) was a colorless oil (3.04 g, 35%).1H NMR (400 MHz, CDCl3) δ 2.33 (s, 3H, CH3), 2.61 (s, 3H, CH3), 6.91 (d, J = 5.0 Hz, 1H, pyHmeta), 8.18 (d, J = 5.0 Hz, 1H, 2995, 2958, 2922, 1585, 1437, 1389, 1123, 1024, 916, 825; HRMS (ESI+) m/z calcd for C7H9BrN [M+H]+ 185.9913, found 185.9917.
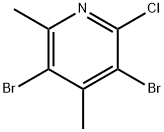
915946-47-3
9 suppliers
inquiry

29976-20-3
39 suppliers
$54.00/100mg
Yield:29976-20-3 83%
Reaction Conditions:
with phosphorus;hydrogen iodide for 16 h;Heating;
References:
Blachut, Dariusz;Czarnocki, Zbigniew;Wojtasiewicz, Krystyna [Synthesis,2006,# 17,p. 2855 - 2864]

108-47-4
229 suppliers
$9.00/1g

27063-92-9
90 suppliers
$65.00/50mg

29976-20-3
39 suppliers
$54.00/100mg

27063-93-0
102 suppliers
$37.00/100mg

857429-63-1
1 suppliers
inquiry

29976-20-3
39 suppliers
$54.00/100mg
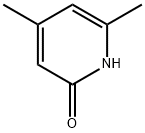
16115-08-5
91 suppliers
$11.00/100mg

29976-20-3
39 suppliers
$54.00/100mg