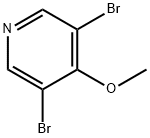
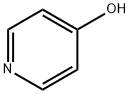
3,5-DibroMo-4-Methoxy-pyridine synthesis
- Product Name:3,5-DibroMo-4-Methoxy-pyridine
- CAS Number:25813-24-5
- Molecular formula:C6H5Br2NO
- Molecular Weight:266.92

67-56-1
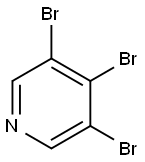
2457-48-9

25813-24-5
Sodium hydride (60% dispersed in mineral oil, 114 mg, 2.85 mmol) was slowly added to a mixed solution of methanol (0.115 mL, 2.85 mmol) and tetrahydrofuran (0.5 mL) at 0 °C. The reaction mixture was stirred at 0 °C for 15 min before 3,4,5-tribromopyridine (600 mg, 1.900 mmol) was added. Subsequently, the reaction mixture was warmed to room temperature and stirred continuously for 19 hours. Upon completion of the reaction, the reaction mixture was diluted with water (3 mL) and extracted with ethyl acetate (2 x 10 mL). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 3,5-dibromo-4-methoxypyridine (0.480 g, 1.798 mmol, 95% yield) as a beige solid. Mass spectrum (electrospray ionization): m/z = 267.9 [M + H]+. 1H NMR (400 MHz, methanol-d4) δ 8.68 (2H, s), 3.99 (3H, s).

67-56-1
787 suppliers
$7.29/5ml-f

2457-48-9
95 suppliers
$43.79/250mg

25813-24-5
51 suppliers
$15.00/250mg
Yield:25813-24-5 95%
Reaction Conditions:
Stage #1:methanol with sodium hydride in tetrahydrofuran at 0; for 0.25 h;
Stage #2:3,4,5-tribromopyridine in tetrahydrofuran at 20; for 19 h;
Steps:
31
To a solution of MeOH (0.115 mL, 2.85 mmol) and THF (0.5 mL) at 0 °C was added sodium hydride (60% dispersion, 1 14 mg, 2.85 mmol). After stirring for 15 min, Intermediate 3 IB (600 mg, 1.900 mmol) was added, and the resulting mixture was stirred at room temperature for 19 hours, diluted with water (3 mL) and extracted with EtOAc (2 x 10 mL). The combined organics were washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to yield Intermediate 31 (0.480 g, 1.798 mmol, 95% yield) as a beige color solid. MS (ES): m/z=267.9 [M+H]+. XH NMR (400 MHz, MeOD) δ ppm 8.68 (2 H, s), 3.99 (3 H, s).
References:
BRISTOL-MYERS SQUIBB COMPANY;AUSTIN, Joel, Francis;SHARMA, Lisa, S.;BALOG, James, Aaron;HUANG, Audris;VELAPARTHI, Upender;DARNE, Chetan, Padmakar;SAULNIER, Mark, George WO2012/15723, 2012, A1 Location in patent:Page/Page column 93
25813-25-6
79 suppliers
$29.00/1g

124-41-4
724 suppliers
$12.00/25g

25813-24-5
51 suppliers
$15.00/250mg
13626-17-0
114 suppliers
$5.00/100mg

124-41-4
724 suppliers
$12.00/25g

25813-24-5
51 suppliers
$15.00/250mg

25813-25-6
79 suppliers
$29.00/1g

74-88-4
357 suppliers
$15.00/10g

25813-24-5
51 suppliers
$15.00/250mg
626-64-2
532 suppliers
$6.00/5g

25813-24-5
51 suppliers
$15.00/250mg