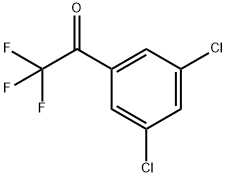
3',5'-DICHLORO-2,2,2-TRIFLUOROACETOPHENONE synthesis
- Product Name:3',5'-DICHLORO-2,2,2-TRIFLUOROACETOPHENONE
- CAS Number:130336-16-2
- Molecular formula:C8H3Cl2F3O
- Molecular Weight:243.01
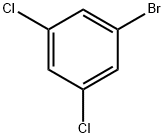
19752-55-7
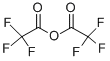
407-25-0

130336-16-2
Under nitrogen protection, tert-butyllithium (1.3 M in pentane, 17.8 mL, 23.2 mmol) was slowly added dropwise to a solution of 3,5-dichloro-1-bromobenzene (5.0 g, 22.1 mmol) in tetrahydrofuran (50 mL) over a period of 30 min, followed by continuing the reaction for 2 h at -78 °C. Upon completion of the reaction, trifluoroacetic anhydride (2.56 g, 12.2 mmol) was slowly added dropwise to the reaction mixture and stirring was continued for 2 hours at -78 °C. The cooling bath was removed and the reaction mixture was allowed to warm slowly to room temperature and stirring was continued at room temperature for 2.5 hours. When the reaction was terminated, saturated ammonium chloride solution (50 mL) was added. The reaction mixture was extracted with ether (3 x 50 mL) and the organic phases were combined and washed with saturated brine (20 mL x 2). The organic phase was separated, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure, and purified by distillation under reduced pressure to give the colorless transparent liquid 3',5'-dichloro-2,2,2-trifluoroacetophenone (2.58 g, 48% yield).
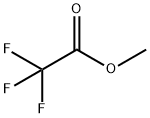
431-47-0
292 suppliers
$10.00/5g

19752-55-7
397 suppliers
$8.00/10g

130336-16-2
367 suppliers
$7.00/100mg
Yield:130336-16-2 75.7%
Reaction Conditions:
Stage #1:1-bromo-3,5-dichlorobenzene with TurboGrignard in tetrahydrofuran at 20 - 25; for 1.75 h;
Stage #2:trifluoroacetic acid-methyl ester in tetrahydrofuran at -10 - 20; for 0.2 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at -10 - 0; for 0.5 h;
Steps:
3 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethanone
To a solution of 1-bromo-3,5-dichlorobenzene (25.0 g, 1 10.6 mmol) in 400 ml THF at room temperature, isopropyl magnesium chloride lithium chloride complex (85.0 ml, 1.3M THF, 1 10.6 mmol) was added dropwise at room temperature over a period of 15 minutes while holding the reaction temperature between 20°C and 25°C. After the addition was complete, the reaction was allowed to stir 1.5 hours at room temperature. The reaction solution was then cooled to -5 °C to -10 °C with ice/MeOH. Methyltrifluoroacetate (12.23 ml, 121 .6 mmol) in 20 ml THF was added dropwise to the reaction solution while maintaining the reaction temp below 0 °C (~ 30 min). The reaction was stirred at -5 °C for 0.5 hr then was allowed to warm to room temperature and stir for 1.5 hrs. The reaction was cooled again to -5 °C to -10 °C then 73.7 ml 6M HCI diluted to 150 ml total volume water was added dropwise while keeping the temperature below 0 °C. Once the addition was complete, the reaction was stirred 0.5 hr at 0 °C then was allowed to warm to room temperature. Excess water was added and the resulting organic layer that separated was drawn off. The aqueous layer was washed repeatedly with DCM. The combined DCM washes and recovered organic layer were dried over sodium sulfate and concentrated to yield 90 g of an oil. The crude material was then passed through a silica gel plug (neat heptane to neat DCM) and purified by vacuum distillation (3 torr, product fractions recovered froml 12 °C to 125 °C) to provide 20.3 g product (75.7% yield). 1 H NMR (400 MHz, CHLOROFORM-d) δ ppm 7.71 (s, 1 H) 7.93 (s, 2 H). IR 1728.1 cm"1
References:
AVISTA PHARMA SOLUTIONS, INC.;SPEAKE, Jason, D. WO2018/9751, 2018, A1 Location in patent:Page/Page column 44-45

19752-55-7
397 suppliers
$8.00/10g

407-25-0
470 suppliers
$9.00/1g

130336-16-2
367 suppliers
$7.00/100mg
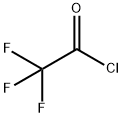
354-32-5
130 suppliers
$55.00/10g

19752-55-7
397 suppliers
$8.00/10g

130336-16-2
367 suppliers
$7.00/100mg
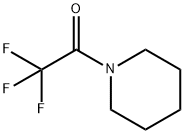
340-07-8
79 suppliers
$6.00/1g

19752-55-7
397 suppliers
$8.00/10g

130336-16-2
367 suppliers
$7.00/100mg
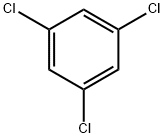
108-70-3
256 suppliers
$19.89/442235
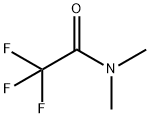
1547-87-1
147 suppliers
$6.00/1g

130336-16-2
367 suppliers
$7.00/100mg