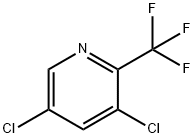
3,5-Dichloro-2-(trifluoromethyl)pyridine synthesis
- Product Name:3,5-Dichloro-2-(trifluoromethyl)pyridine
- CAS Number:7655-72-3
- Molecular formula:C6H2Cl2F3N
- Molecular Weight:215.99
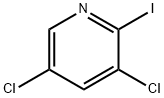
1214350-54-5
9 suppliers
inquiry
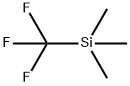
81290-20-2
416 suppliers
$13.00/1g

7655-72-3
22 suppliers
$104.00/1g
Yield:7655-72-3 92%
Reaction Conditions:
Stage #1: (trifluoromethyl)trimethylsilanewith potassium fluoride;copper(l) iodide in 1-methyl-pyrrolidin-2-one at 50; for 0.75 h;
Stage #2: 3,5-dichloro-2-iodopyridine in 1-methyl-pyrrolidin-2-one at 50;
Steps:
3,5-dichloro-2-(trifluoromethyl)pyridine
KF (87.18mg, 1.5 mmol) and Cul (285.80 mg, 1.5 mmol) were thoroughly mixed before being heated under vacuum (1 mm Hg) with the flame of a Bunsen burner with gentle shaking until an homogeneous greenish color was obtained. NMP (25 mL), trimethyl(trifluoromethyl)silane (213.88 mg, 1.5 mmol). The mixture was stirred at 50 °C for 45 min. 3,5-dichloro-2-iodopyridine (2250 mg, 7.45 mmol) was added. The mixture was stirred at 50°C overnight. The reaction was followed by GC-MS, which indicated formed product. Water (50 mL) was added to mixture and extracted with Ethyl acetate (5 mL x 3). The combined organic layers were washed with brine and evaporated to afford crude product, which was purified by biotage (EtOAc/PE=l% ~ 50%, ISCO 12 g, 10 mL/min, normal phase silica geluv 254) to afford the target compound (198 mg, 92% yield) as brown oil.
NMR (400 MHz, MeOD) δ 8.66 (d, J= 2.0 Hz, 1H), 8.34 - 8.24 (m, 1H).
GC-MS m/z calcd for [C6H2C12F3N]: 215.0; found: 215.0.
References:
WO2018/11093,2018,A1 Location in patent:Page/Page column 77
14482-51-0
273 suppliers
$10.00/5g

7655-72-3
22 suppliers
$104.00/1g
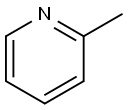
109-06-8
419 suppliers
$16.00/25g

7655-72-3
22 suppliers
$104.00/1g