3,5-DIFLUORO-4-HYDROXY-BENZONITRILE synthesis
- Product Name:3,5-DIFLUORO-4-HYDROXY-BENZONITRILE
- CAS Number:2967-54-6
- Molecular formula:C7H3F2NO
- Molecular Weight:155.1
![Benzaldehyde, 3,5-difluoro-4-hydroxy-, oxime, [C(E)]-](/CAS/20240320/GIF/960287-97-2.gif)
960287-97-2

2967-54-6
The general procedure for the synthesis of 3,5-difluoro-4-hydroxybenzonitrile from 3,5-difluoro-4-hydroxybenzaldehyde oxime is as follows: to a solution of tetrahydrofuran (THF, 10 mL) containing the aldoxime (0.01 mol) was added T3P (15 mol%, 50% solution in ethyl acetate (EtOAc)). The reaction mixture was stirred at room temperature for 1-2 h under nitrogen protection. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming the completion of the reaction, the solvent was removed under reduced pressure. The residue was diluted with distilled water (20 mL) and subsequently extracted with ethyl acetate (2 x 20 mL). The organic phases were combined and washed sequentially with saturated sodium bicarbonate (NaHCO3) solution (10 mL) and brine. The organic phase was dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to afford the target product 3,5-difluoro-4-hydroxybenzonitrile of high purity.
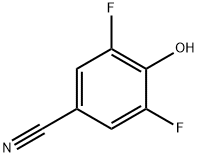
104197-15-1
79 suppliers
$45.00/250mg

2967-54-6
73 suppliers
$10.00/250mg
Yield: 80.6%
Reaction Conditions:
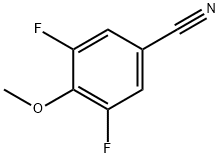
Stage #1:3,5-difluoro-4-methoxybenzonitrile with boron tribromide in dichloromethane at -78 - 20;
Stage #2: with water in dichloromethane at 0;
Steps:
B.iv
(iv) 3,5-Difluoro-4-hydroxybenzonitrileBBr3 (6.61 g, 0.0264 mol) was added to 3,5-difluoro-4-methoxybenzonitrile (1.5 g, 0.0088 mol; see step (iii) above) in dichloromethane (15 mL) at -780C. Stirring was continued at room temperature overnight under a nitrogen atmosphere. The reaction mixture was then quenched with ice water and extracted with dichloromethane. The organic layer was washed with water and brine, and dried over sodium sulfate. Solvent evaporation under reduced pressure yielded 1.1 g (80.6%) of the sub-title compound as a grey solid.
References:
ASTRAZENECA AB WO2006/135316, 2006, A1 Location in patent:Page/Page column 55
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2967-54-6
73 suppliers
$10.00/250mg
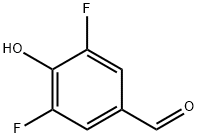
118276-06-5
169 suppliers
$14.00/250mg

2967-54-6
73 suppliers
$10.00/250mg

23382-09-4
0 suppliers
inquiry
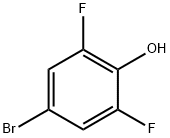
104197-13-9
242 suppliers
$6.00/1g

2967-54-6
73 suppliers
$10.00/250mg
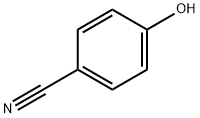
767-00-0
582 suppliers
$6.00/25g
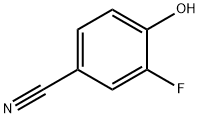
405-04-9
198 suppliers
$6.00/1g

2967-54-6
73 suppliers
$10.00/250mg