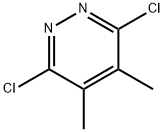
3,6-Dichloro-4,5-dimethylpyridazine synthesis
- Product Name:3,6-Dichloro-4,5-dimethylpyridazine
- CAS Number:34584-69-5
- Molecular formula:C6H6Cl2N2
- Molecular Weight:177.03
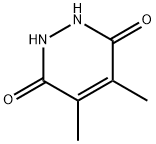
5754-17-6

34584-69-5
General procedure for the synthesis of 3,6-dichloro-4,5-dimethylpyridazine from 4,5-dimethylpyridazine-3,6-dione: A mixture of 6-hydroxy-4,5-dimethyl-2H-pyridazin-3-one (2.56 g, 18 mmol), phosphorus trichloride (8 mL), and diisopropylethylamine (4 mL) was placed in a microwave reactor (Biotage MW oven) and The reaction was stirred at 160 °C for 20 min. Upon completion of the reaction, the solvent was partially evaporated under reduced pressure and the residue was poured into a mixture of cold water, saturated sodium bicarbonate solution and methylene chloride. The mixture was alkalized with saturated sodium bicarbonate solution until no CO2 gas was released. The organic layer was separated, dried over anhydrous sodium sulfate, filtered and the solvent concentrated under reduced pressure. The residue was purified by column chromatography (eluent: dichloromethane/heptane, ratio tapered from 1/1 to 10/0) to afford the target product 3,6-dichloro-4,5-dimethylpyridazine (D9) (1.7 g, 53% yield) as a solid. Elemental analysis: theoretical value 176 for C6H6Cl2N2; measured value 177 (MH+).

5754-17-6
99 suppliers
$16.00/250mg

34584-69-5
137 suppliers
$8.00/1g
Yield:34584-69-5 53%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate at 160; for 0.333333 h;Microwave irradiation;
Steps:
9
Description 9; 3,6-Dichloro-4,5-dimethyl-pyridazine (D9); A mixture of 6-hydroxy-4,5-dimethyl-2H-pyridazin-3-one (2.56 g, 18 mmol) (prepared by a procedure similar to that described in WO 99/36407), phosphorus oxychloride (8 ml) and diisopropylethylamine (4 ml) was stirred at 160 0C for 20 min., under microwave irradiation (Biotage MW-oven). The solvent was then partially evaporated in vacuo and remaining material poured into a mixture of cold water, saturated sodium hydrogen carbonate and dichloromethane. The mixture was then basified with portions of sodium hydrogen carbonate until there was no more CO2 evolution. The organic layer was separated, dried (Na2SO4), filtered and the solvent evaporated in vacuo. The residue was purified by column chromatography (dichloromethane / heptane 1/1 to 10/0) to yield D9 (1.7 g, 53 %) as a solid. C6H6Cl2N2 requires 176; Found 177 (MH+)
References:
WO2008/68277,2008,A1 Location in patent:Page/Page column 21

5754-17-6
99 suppliers
$16.00/250mg

34584-69-5
137 suppliers
$8.00/1g
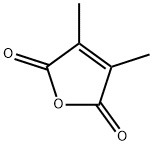
766-39-2
250 suppliers
$10.00/5g

34584-69-5
137 suppliers
$8.00/1g

63751-07-5
0 suppliers
inquiry

34584-69-5
137 suppliers
$8.00/1g