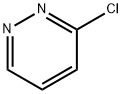
3-CHLOROPYRIDAZINE synthesis
- Product Name:3-CHLOROPYRIDAZINE
- CAS Number:1120-95-2
- Molecular formula:C4H3ClN2
- Molecular Weight:114.53
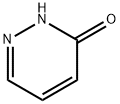
504-30-3

1120-95-2
Step A: 3-(2H)-pyridazinone (5.0 g, 52.0 mmol) was mixed with phosphorus trichloride (17 mL, 179 mmol) and reacted at 85 °C for 4.5 hours. Upon completion of the reaction, the mixture was slowly poured into 400 g of ice-water mixture and the pH was adjusted to >10 with 50% NaOH solution, followed by extraction with ethyl acetate (4×). The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography (Hak-Pak, eluent ratio 1:1 hexane/ethyl acetate) to afford 3-chloropyridazine (2.8 g, 46% yield).

504-30-3
238 suppliers
$6.00/1g

1120-95-2
137 suppliers
$50.00/100 mg
Yield:1120-95-2 92%
Reaction Conditions:
with trichlorophosphate at 60; for 1.5 h;
Steps:
118
Preparation 118: 3-Chloro-pyridazine (Prepi 18); A mixture of pyridazin-3-ol (1.9 g, 19.8 mmol) in POCI3 (19 ml) was heated to 600C for 90 minutes. After cooling to room temperature, the reaction was quenched in ice/water and neutralized with solid NaHCO3. The product was extracted with ethyl acetate, the organic phase was washed with brine, dried (Na2SO4) and evaporated to give 2.1 g of the title compound as a brown solid (92% yield). MS (ES) (m/z): 115.1 [M+H]+.
References:
Glaxo Group Limited WO2007/113232, 2007, A1 Location in patent:Page/Page column 122

504-30-3
238 suppliers
$6.00/1g

1120-95-2
137 suppliers
$50.00/100 mg