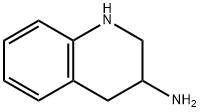
(+/-)-3-AMINO-1,2,3,4-TETRAHYDROQUINOLINE synthesis
- Product Name:(+/-)-3-AMINO-1,2,3,4-TETRAHYDROQUINOLINE
- CAS Number:40615-02-9
- Molecular formula:C9H12N2
- Molecular Weight:148.21
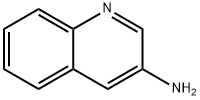
580-17-6

40615-02-9
GENERAL METHOD: In a 1.5 mL reaction vial, tris(pentafluorophenyl)borane [B(C6F5)3] (0.025 mmol, 5.0 mol%) was dissolved in chloroform (0.60 mL), followed by the addition of diethylsilane (1.75 mmol, 3.5 equiv). After a short shaking, quinoline derivative (1a-p, 0.50 mmol, 1.0 eq.) was added to the above catalyst solution under argon protection. The reaction mixture was stirred at 25-65 °C for 6-24 h (for 1a-h) or at 25-100 °C for 2-24 h (for 1i-p) and subsequently cooled to room temperature. The reaction mixture was concentrated under reduced pressure to give the crude product. The crude product was treated with an ether solution (7 mL) of 0.25 N HCl and stirred at room temperature for 1 h to give a solid residue, which was subsequently washed with ether. The solid residue was dissolved or suspended in methanol (1.0 mL) and neutralized with sodium carbonate monohydrate (Na2CO3-H2O, 0.5 g) at 0 °C. After stirring for 2 h, methanol was removed under reduced pressure and the neutralized reaction residue was dissolved in dichloromethane (CH2Cl2) and washed sequentially with brine (5 mL) and water (5 mL). The crude product was isolated from the organic phase of the dichloromethane solution and finally purified by silica gel column chromatography using ethyl acetate/hexane (1:9 for 2a-h; 3:7 for 2i-p) as eluent to give the target product 2a-p.

580-17-6
202 suppliers
$13.43/1gm:

40615-02-9
53 suppliers
$15.00/100mg
Yield: 68%
Reaction Conditions:
Stage #1:3-aminoquinoline with H2SiEt2;tris(pentafluorophenyl)borate in chloroform at 100; for 24 h;Inert atmosphere;
Stage #2: with hydrogenchloride in diethyl ether at 20; for 1 h;
Steps:
III. Reduction of Substituted Quinolines and Other N-Heteroarenes (Schemes 2-3)
General procedure: In a 1.5 mL reaction vial, B(C6F5)3 (0.025 mmol, 5.0 mol %) was dissolved in chloroform (0.60 mL), towhich diethylsilane (1.75 mmol, 3.5 equiv) was added. After shaking briefly, quinolines (1a-p, 0.50 mmol, 1.0equiv) was subsequently added to the above catalyst solution under argon atmosphere. The reaction mixturewas stirred at 25-65 oC for 6-24 h for the reaction of 1a-h, and at 25-100 oC for 2-24 h for the reaction of 1i-p,then allowed to cool down to room temperature and concentrated under reduced pressure to give the crudeproduct. This reaction mixture was then treated with 0.25 N HCl ethereal solution (7 mL) and stirred at roomtemperature for 1 h to give the solid residue, which was subsequently washed with ether. The solid residue wasthen dissolved or suspended in MeOH (1.0 mL) and neutralized with Na2CO3·H2O (0.5 g) at 0 oC. After stirringfor 2 h, MeOH was removed under reduced pressure, and the neutralized reaction residue was dissolved inCH2Cl2 and washed with brine (5 mL) and water (5 mL). The crude product was then obtained from the organicphase of CH2Cl2 solution and finally purified by column chromatography on silica gel to give 2a-h(EtOAc/Hexane = 1/9) and 2i-p (EtOAc/Hexane = 3/7).
References:
Gandhamsetty, Narasimhulu;Park, Sehoon;Chang, Sukbok [Synlett,2017,vol. 28,# 18,p. 2396 - 2400] Location in patent:supporting information