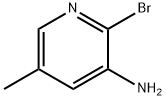
3-Amino-2-bromo-5-methylpyridine synthesis
- Product Name:3-Amino-2-bromo-5-methylpyridine
- CAS Number:34552-14-2
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
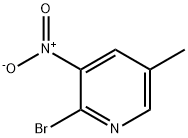
23056-46-4

34552-14-2
Step A: Preparation of 2-bromo-5-methylpyridin-3-amine: acetic acid (5 mL) was slowly added dropwise to iron powder (1.11 g, 19.8 mmol) and the mixture was heated to 80 °C. Subsequently, a solution of acetic acid (5 mL) of 2-bromo-5-methyl-3-nitropyridine (1.0 g, 4.61 mmol) was added dropwise over a period of 20 min, and stirring was continued for 30 min after completion of the dropwise addition. The reaction mixture was stirred at room temperature for 16 hours, then diluted with ethyl acetate (20 mL) and filtered through diatomaceous earth (Celite). The diatomaceous earth pad was washed well with ethyl acetate, the filtrates were combined and concentrated under reduced pressure. The residue was slowly added to saturated sodium bicarbonate solution (50 mL), followed by addition of solid sodium bicarbonate in batches until the acetic acid was completely neutralized. The mixture was extracted with ethyl acetate (3 x 30 mL), and the combined organic phases were washed with brine (20 mL), dried over anhydrous sodium sulfate, filtered and concentrated to afford 2-bromo-5-methylpyridin-3-amine (799 mg, 93% yield) as a bright yellow crystalline solid, which darkened to a greenish color after drying under vacuum. Mass spectrum (APCI) m/z = 188.9 (M + H).

23056-46-4
148 suppliers
$9.00/1g

34552-14-2
127 suppliers
$16.00/1g
Yield: 93%
Reaction Conditions:
with iron;acetic acid at 20 - 80; for 18.8333 h;
Steps:
30.A Step A: Preparation of 2-bromo-5-methylpyridin-3-amine
Example 30 1 -((3 S,4R)-4-(3 ,4-difluorophenyl)- 1 -(2-methoxyethyl)pyrrolidin-3-yl)-3 -(5-methyl-2-phenylpyridin-3 -yl)urea1006131 Step A: Preparation of 2-bromo-5-methylpyridin-3-amine: Acetic acid (5 mL) was added dropwise to iron powder (1.11 g, 19.8 mmol) and the mixture was warmed to 80 °C. A solution of 2-bromo-5-methyl-3-nitropyridine (1.0 g, 4.61 mmol) in AcOH (5 mL) was then added dropwise over 20 minutes and the mixture stirred for a further 30 minutes. The mixture was stirred at ambient temperature for 16 hours then diluted with EtOAc (20 mL) and filtered through Celite. The Celite pad was washed well with EtOAc and the filtrate concentrated under vacuum. The residue was slowly treated with saturated NaHCO3 solution (50 mL) followed by portions of solid NaHCO3 until all the AcOH was neutralized. The mixture was then extracted with EtOAc (3 x 30 mE) and the combined organic phases were washed with brine (20 mE), dried over Na2SO4, filtered and concentrated to afford 2-bromo-5-methylpyridin-3-amine (799 mg, 93% yield) as a bright yellow crystalline solid which darkened to a green color after drying under vacuum. MS (apci) mlz = 188.9 (M+H).
References:
ARRAY BIOPHARMA INC.;ALLEN, Shelley;ANDREWS, Steven, Wade;BLAKE, James, F.;BRANDHUBER, Barbara, J.;CONDROSKI, Kevin, Ronald;HAAS, Julia;JIANG, Yutong;KERCHER, Timothy;KOLAKOWSKI, Gabrielle, R.;WINSKI, Shannon, L. WO2014/78378, 2014, A1 Location in patent:Paragraph 00613