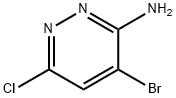
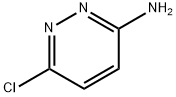
3-Amino-4-bromo-6-chloropyridazine synthesis
- Product Name:3-Amino-4-bromo-6-chloropyridazine
- CAS Number:446273-59-2
- Molecular formula:C4H3BrClN3
- Molecular Weight:208.44
5469-69-2

446273-59-2
Example I (1) Bromine (9.71 g, 3.15 mL, 60.75 mmol) was slowly added dropwise to a mixed solution containing 3-amino-6-chloropyridazine (7.87 g, 60.75 mmol), methanol (115 mL) and sodium bicarbonate (10.22 g, 121.67 mmol). The reaction mixture was stirred at room temperature for 16 hours and then filtered. Water (500 mL) was added to the filtrate and subsequently extracted with ethyl acetate (3 × 500 mL). The organic layers were combined and concentrated under reduced pressure. The resulting residue was purified by fast chromatography using 1:1 hexane/ethyl acetate as eluent, resulting in 5.40 g of 3-amino-4-bromo-6-chloropyridazine in 43% yield.

5469-69-2
376 suppliers
$6.00/10g

446273-59-2
241 suppliers
$5.00/250mg
Yield:446273-59-2 99%
Reaction Conditions:
with bromine;sodium hydrogencarbonate in methanol at 0 - 20; for 16 h;
Steps:
29-2.A Step A:
6-Chloropyridazin-3-amine (50 g, 388 mmol) and NaHC03 (65 g, 775 mmol) were combined in MeOH (500 mL). To the mixture was added Br2 (30 mL, 580 mmol) dropwise at 0 °C. The mixture was stirred at room temperature for 16 h. One half of the volume of solvent was removed under reduced pressure. The remaining was poured into ice water. The solid formed was collected and dried to yield 4-bromo-6-chloropyridazin-3-amine (80 g, 99%). MS m/z 207.9 [M+H]+.
References:
WO2018/226622,2018,A1 Location in patent:Page/Page column 184; 185